AMH decline during chemotherapy reflects breast cancer cell DNA damage response (DDR) proficiency: the ONCO AMH1 pilot study
- PMID: 40220108
- PMCID: PMC12167210
- DOI: 10.1007/s10815-025-03475-9
AMH decline during chemotherapy reflects breast cancer cell DNA damage response (DDR) proficiency: the ONCO AMH1 pilot study
Abstract
Purpose: The impact of a germline BRCA1/2 pathogenic variant (gBRCApv) on baseline or late post-treatment AMH concentrations in breast cancer patients has been extensively studied, yielding mixed conclusions. However, whether the AMH decline during neo-adjuvant chemotherapy reflects differences in chemotherapy susceptibility between gBRCApv carriers and non-carriers remains unexplored.
Methods: A monocentric, retrospective, longitudinal study was conducted on breast cancer patients carrying a gBRCApv (n = 12) or wild-type (WT) (n = 35) who received a neo-adjuvant sequential chemotherapy (CT) with anthracyclines followed by taxanes. Serum AMH levels were measured at baseline (AMH0) and at three time points during CT by a hypersensitive assay. Tumor size change was assessed via imaging. The impact of genetic status on AMH decline was evaluated using a linear mixed model with post hoc analysis.
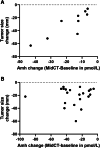
Results: The change of AMH concentrations from baseline to the end of CT tended to be influenced by the genetic status (BRCA * time interaction, p = 0.058). The slope between AMH0 and the end of anthracyclines (after log transformation) was steeper in gBRCApv than in WT patients (mean (SE): - 5.54 (0.63) vs - 3.97 (0.62); p = 0.023). Tumor size change was positively and significantly correlated with the change in AMH levels (AMH MidCT-AMH0) in gBRCApv patients (r = 0.93, p < 0.001) but not in WT patients (r = - 0.05; p = 0.84).
Conclusion: Germline BRCA1/2 status influences AMH decline during neo-adjuvant CT with drugs inducing DNA lesions. AMH decay is positively related to tumor size change assessed by imaging in gBRCApv patients. However, no conclusions can be drawn regarding the relationship with treatment response assessed by histological criteria.
Keywords: BRCA1/2 germline pathogenic variant; AMH; Breast cancer; Chemotherapy; DNA damage response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: ONCOAMH1 study has been reviewed and approved by the Commission for Clinical Studies of the Centre Oscar Lambret (Lille, France) under the reference CEC- 2023–022. This study complies with the Reference Methodology MR004 of the CNIL (Commission Nationale de l’Informatique & des Libertés, Paris, France). Conflict of interest: The authors declare no competing interests.
Figures

Similar articles
-
Anti-Müllerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations.Hum Reprod. 2016 May;31(5):1126-32. doi: 10.1093/humrep/dew044. Hum Reprod. 2016. PMID: 27094481 Free PMC article.
-
Toxicity of (neo)adjuvant chemotherapy for BRCA1- and BRCA2-associated breast cancer.Breast Cancer Res Treat. 2016 Apr;156(3):557-566. doi: 10.1007/s10549-016-3777-0. Epub 2016 Apr 9. Breast Cancer Res Treat. 2016. PMID: 27060914 Free PMC article.
-
A prospective longitudinal analysis of the predictors of amenorrhea after breast cancer chemotherapy: Impact of BRCA pathogenic variants.Cancer Med. 2023 Sep;12(18):19225-19233. doi: 10.1002/cam4.6527. Epub 2023 Sep 12. Cancer Med. 2023. PMID: 37698031 Free PMC article.
-
Hematologic toxicities of chemotherapy in breast and ovarian cancer patients carrying BRCA1/BRCA2 germline pathogenic variants. A single center experience and review of the literature.Fam Cancer. 2023 Jul;22(3):283-289. doi: 10.1007/s10689-023-00331-6. Epub 2023 Apr 29. Fam Cancer. 2023. PMID: 37119509 Free PMC article. Review.
-
Navigating the body of literature assessing BRCA1/2 mutations and markers of ovarian function: a systematic review and meta-analysis.J Assist Reprod Genet. 2020 May;37(5):1037-1055. doi: 10.1007/s10815-020-01745-2. Epub 2020 Mar 24. J Assist Reprod Genet. 2020. PMID: 32212026 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

