Biosynthesis of ergothioneine: current state, achievements, and perspectives
- PMID: 40220171
- PMCID: PMC11993508
- DOI: 10.1007/s00253-025-13476-4
Biosynthesis of ergothioneine: current state, achievements, and perspectives
Abstract
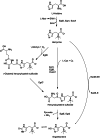
Ergothioneine (EGT) is a derivative of the amino acid L-histidine that is well known for its strong antioxidant properties. Recent studies on the functional characterization of EGT in both in vivo and in vitro systems have demonstrated its potential applications in pharmaceuticals, food, and cosmetics. The growing demand for EGT in novel applications necessitates the development of safe and cost-effective mass production technologies. Consequently, microbial fermentation for EGT biosynthesis has attracted significant attention. This review focuses on the biosynthesis of EGT via microbial fermentation, explores its biosynthetic mechanisms, and summarizes the latest advancements for industrial EGT production using engineered microbial strains. KEY POINTS: • Ergothioneine (EGT) is an L-histidine derivative with strong antioxidant property. • Recent studies have revealed certain groups of microbes produce EGT naturally. • Superior EGT producers by genetic modification have been created.
Keywords: Antioxidant; Basidiomycetes; Biosynthesis; Ergothioneine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors. Conflict of interest: The authors declare no competing interests.
Figures

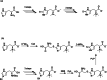
Similar articles
-
Ergothioneine: new functional factor in fermented foods.Crit Rev Food Sci Nutr. 2024 Jul;64(21):7505-7516. doi: 10.1080/10408398.2023.2185766. Epub 2023 Mar 9. Crit Rev Food Sci Nutr. 2024. PMID: 36891762 Review.
-
Research progress and development prospects of microbial synthesis of ergothioneine.World J Microbiol Biotechnol. 2025 May 26;41(6):184. doi: 10.1007/s11274-025-04415-6. World J Microbiol Biotechnol. 2025. PMID: 40415044 Review.
-
Fermentative Production of Ergothioneine by Exploring Novel Biosynthetic Pathway and Remodulating Precursor Synthesis Pathways.J Agric Food Chem. 2024 Jun 26;72(25):14264-14273. doi: 10.1021/acs.jafc.4c03348. Epub 2024 Jun 11. J Agric Food Chem. 2024. PMID: 38860833
-
Recent Strategies for the Biosynthesis of Ergothioneine.J Agric Food Chem. 2021 Nov 24;69(46):13682-13690. doi: 10.1021/acs.jafc.1c05280. Epub 2021 Nov 10. J Agric Food Chem. 2021. PMID: 34757754
-
Successful biosynthesis of natural antioxidant ergothioneine in Saccharomyces cerevisiae required only two genes from Grifola frondosa.Microb Cell Fact. 2020 Aug 18;19(1):164. doi: 10.1186/s12934-020-01421-1. Microb Cell Fact. 2020. PMID: 32811496 Free PMC article.
References
-
- Bao HND, Ushio H, Ohshima T (2008) Antioxidative activity and antidiscoloration efficacy of ergothioneine in mushroom (Flammulina velutipes) extract added to beef and fish meats. J Agric Food Chem 56:10032–10040 - PubMed
-
- Bello MH, Barrera-Perez V, Morin D, Epstein L (2012) The Neurospora crassa mutant NcDEgt-1 identifies an ergothioneine biosynthetic gene and demonstrates that ergothioneine enhances conidial survival and protects against peroxide toxicity during conidial germination. Fungal Genet Biol 49:160–172 - PubMed
-
- Burn R, Misson L, Meury M, Seebeck FP (2017) Anaerobic origin of ergothioneine. Angew Chem Int Ed 56:12508–12511 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

