3D virtual surgical planning of fractures in hip arthrodesis: a systematic review, case series and recommendations for treatment
- PMID: 40220176
- PMCID: PMC11993491
- DOI: 10.1007/s00590-025-04283-8
3D virtual surgical planning of fractures in hip arthrodesis: a systematic review, case series and recommendations for treatment
Abstract
Purpose: Fractures through an arthrodesed hip are rare and challenging. The aim of the study is (1) to explore whether 3D-planned percutaneous screw fixation of fractures in hip arthrodesis is a viable minimally invasive surgical option for geriatric patients and (2) to standardize surgical treatment by providing a comprehensive overview of the literature and propose a treatment algorithm.
Methods: We presented a case series of patients with an acute fracture in a previous hip arthrodesis treated in a level 1 trauma centre in 2024. Furthermore, we conducted a systematic review on fractures in hip arthrodesis from 1970 to 2023.
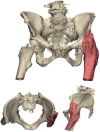
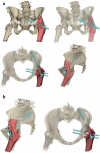
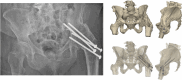
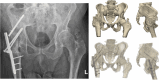
Results: We presented three cases treated for a fracture in an arthrodesed hip. Two patients with a proximal/medial fracture to the acetabulum were operated with 3D-planned percutaneous cannulated screws, and one patient with an intertrochanteric fracture was operated with a DHS system. The systematic review resulted in an overview of 16 case series on fractures in hip arthrodesis treated with various surgical techniques, each with its pros and cons; cannulated screws, DHS system, intramedullary nailing and plate osteosynthesis.
Conclusion: Acute fractures in arthrodesed hips in fragile geriatric patients can be treated minimally invasively with 3D-planned percutaneous screw fixation. This technique is most suitable for femoral neck fracture types. Alternative surgical techniques include DHS, intramedullary nailing, plate osteosynthesis or conversion to total hip arthroplasty, for which a treatment algorithm is provided.
Keywords: 3D; Ankylosis; Hip arthrodesis fracture; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: This study was performed in line with the principles of the Declaration of Helsinki. This study was reviewed, and a waiver was provided by the Medical Ethics Review Committee of the University Medical Center Groningen, No: METc 2017/543. Informed consent was obtained from all individual participants included in the study.
Figures



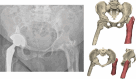



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

