Functional sinonasal outcomes after rescue flap versus double nasoseptal flap in endoscopic trans-sphenoid pituitary surgery: a randomized clinical trial
- PMID: 40220183
- PMCID: PMC12055946
- DOI: 10.1007/s00405-025-09342-8
Functional sinonasal outcomes after rescue flap versus double nasoseptal flap in endoscopic trans-sphenoid pituitary surgery: a randomized clinical trial
Abstract
Objectives: The aim of the current study was to evaluate the functional sinonasal outcomes after rescue flap versus double nasoseptal flap in endoscopic endonasal pituitary surgery.
Methods: This randomized clinical trial was conducted over 1.5 years over 60 patients who underwent endoscopic trans-sphenoid surgery for macroadenomas (more than 2 cm.). the patients were randomly allocated into 2 groups: the rescue flap group, (n = 30) and the double nasoseptal flap group (n = 30). Functional sinonasal outcomes were evaluated in both groups in terms of sinonasal outcome test (SNOT-22), crusting, adhesions, and olfaction.
Results: the sinonasal outcome test (SNOT-22), as well as the olfaction scores were significantly better in the double flap group compared to the rescue flap group. Crusting and adhesions occurred more frequently in the rescue flap group. The nasal stage operative time was significantly longer in the double flap group than the rescue flap group.
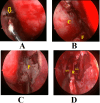
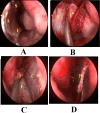
Conclusion: Endoscopic pituitary surgery can adversely affect the sinonasal functions. Double nasoseptal flap technique allows posterior septectomy with bilateral septal mucosa preservation. Although it requires longer operative time than the rescue flap technique, better functional sinonasal outcomes and olfaction scores are achieved.
Keywords: Nasoseptal flap; Outcomes; Pituitary adenoma; Rescue flap; Sinonasal; Trans-sphenoid.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The study was approved by the Mansoura Faculty of Medicine Institutional Research Board (IRB: MD.22.11.721.R1). This randomized clinical trial was registered at ClinicalTrials.gov (NCT06526481). Informed consent: Informed consent was obtained from all individual participants included in the study. Conflict of interest: The authors declare that they have no conflict of interest.
Figures
References
-
- Gstrein NA, Zwicky S, Serra C, Hugelshofer M, Regli L, Soyka MB, Holzmann D, Meerwein CM (2023) Rhinologic outcome of endoscopic transnasal-transsphenoidal pituitary surgery: an institutional series, systematic review, and meta-analysis. Eur Arch Otorhinolaryngol 280(9):4091–4099 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

