Inside the Belly of the Beast: Exploring the Gut Bacterial Diversity of Gonipterus sp. n. 2
- PMID: 40220189
- PMCID: PMC11993490
- DOI: 10.1007/s00248-025-02524-1
Inside the Belly of the Beast: Exploring the Gut Bacterial Diversity of Gonipterus sp. n. 2
Abstract
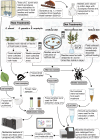
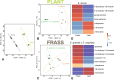
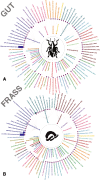
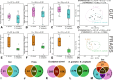
The Eucalyptus snout beetle (Gonipterus sp. n. 2) is a destructive invasive pest of Eucalyptus plantations, responsible for significant defoliation and wood yield losses globally. Native to Australia, this beetle has adapted to thrive on diverse Eucalyptus hosts, overcoming their chemical defences. However, the mechanisms by which Gonipterus tolerates or utilises these plant defence metabolites remain poorly understood. In South Africa, Gonipterus sp. n. 2 poses a significant threat to Eucalyptus plantations by causing extensive defoliation and leading to substantial reductions in growth and wood production. This study investigates the relationship between diet, host Eucalyptus species, and the gut microbiome of Gonipterus sp. n. 2. Using controlled feeding experiments, beetles were reared on artificial, semi-artificial, and natural diets, as well as two Eucalyptus genotypes with distinct secondary metabolite profiles. High-throughput 16S rDNA sequencing and gas chromatography-mass spectrometry (GC-MS) revealed significant shifts in gut bacterial diversity and composition across diets. Natural diets supported the most diverse microbial communities, while artificial diets fostered a homogenised microbiome dominated by opportunistic taxa like Serratia. Host-specific effects were observed in frass microbiota, with substantial biotransformation of monoterpenes into less toxic derivatives. The results highlight the plasticity of Gonipterus gut microbiota, which enables metabolic adaptability and resilience in diverse environments. This microbial flexibility underpins the invasiveness of Gonipterus, emphasising the role of gut symbionts in overcoming host chemical defences. Understanding these interactions offers novel insights for microbiome-targeted pest management strategies, providing a sustainable approach to mitigate the impact of Gonipterus on global Eucalyptus forestry.
Keywords: Eucalyptus snout beetle; Insect-microbe interactions; Invasive pests; Microbiome; Plant metabolites.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Mapondera TS, Burgess T, Matsuki M, Oberprieler RG (2012) Identification and molecular phylogenetics of the cryptic species of the Gonipterus scutellatus complex (Coleoptera: Curculionidae: Gonipterini). Aust J Entomol 51:175–188. 10.1111/j.1440-6055.2011.00853.x - DOI
-
- Schröder ML, Slippers B, Wingfield MJ, Hurley BP (2020) Invasion history and management of Eucalyptus snout beetles in the Gonipterus scutellatus species complex. J Pest Sci 93:11–25. 10.1007/s10340-019-01156-y - DOI
-
- Reis AR, Ferreira L, Tomé M, Araujo C, Branco M (2012) Efficiency of biological control of Gonipterus platensis (Coleoptera: Curculionidae) by Anaphes nitens (Hymenoptera: Mymaridae) in cold areas of the Iberian Peninsula: Implications for defoliation and wood production in Eucalyptus globulus. For Ecol Manage 270:216–222. 10.1016/j.foreco.2012.01.038 - DOI
-
- Cooper PD (2001) What physiological processes permit insects to eat Eucalyptus leaves? Austral Ecol 26:556–562. 10.1046/j.1442-9993.2001.01142.x - DOI
-
- Salehi B, Sharifi-Rad J, Quispe C, Llaique H, Villalobos M, Smeriglio A, Trombetta D, Ezzat SM, Salem MA, Zayed A (2019) Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci Technol 91:609–624. 10.1016/j.tifs.2019.08.003 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

