Efficient and high-density immobilization of animal cells by a microfiber with both swelling and cell adhesion properties and its application to exosome production
- PMID: 40220210
- PMCID: PMC11993475
- DOI: 10.1007/s10529-025-03585-5
Efficient and high-density immobilization of animal cells by a microfiber with both swelling and cell adhesion properties and its application to exosome production
Abstract
Purpose: For high-density cell culture, we studied the development of optimal microfibers (MFs) with a 0.1-10 μm diameter, which due to their large surface area can serve as an immobilization carrier for animal cells. To date, few studies have used MFs as scaffolding for high-density cell culturing.
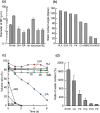
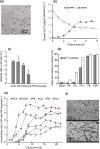
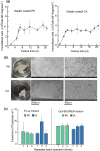
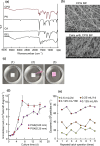
Results: Using six types of nonsoluble synthetic polymers, MF sheets were fabricated by electrospinning. The cellulose acetate, polyketone, and polyvinyl acetate MFs exhibited swelling and water retention capacities. Next, the six types of MF fragments were examined for immobilizing TKD2 mouse vascular endothelial cells. Although most cells were taken into the three MFs characterized by swelling, most leaked from the MFs without adhesion. To solve this, the MF sheets comprising cellulose acetate and polyketones were coated with gelatin. Although the adhesive capacity was enhanced, the swelling capacity decreased and almost all the immobilized mouse cells remained on the sheets' surfaces. Based on these results, we produced a novel MF sheet comprising a gelatin, cellulose acetate, and polyketone mixture (CPG). Since the cells were taken into the MFs by swelling and attached by the gelatin, the CPG fragment immobilized almost all the supplied cells with little loss and reached a high density of 3.2 × 109 MF-g-1, Furthermore, the immobilized cells continuously produced exosomes with a high productivity of 6-7 × 1010 particles ml-1 after either 8 h or 16 h of culturing.
Conclusion: CPG-based MFs are expected to have a wide range of future applications, including exosome production from animal cells.
Keywords: Electrospinning; Exosomes; High-density immobilization; Microfiber; Swelling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: This study did not involve human or animal participants and there was no requirement for consent.
Figures

Similar articles
-
Immobilization of cell-adhesive peptides to temperature-responsive surfaces facilitates both serum-free cell adhesion and noninvasive cell harvest.Tissue Eng. 2004 Jul-Aug;10(7-8):1125-35. doi: 10.1089/ten.2004.10.1125. Tissue Eng. 2004. PMID: 15363169
-
Hydrophilic PCU scaffolds prepared by grafting PEGMA and immobilizing gelatin to enhance cell adhesion and proliferation.Mater Sci Eng C Mater Biol Appl. 2015 May;50:201-9. doi: 10.1016/j.msec.2015.02.015. Epub 2015 Feb 11. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 25746263
-
A promising approach toward efficient isolation of the exosomes by core-shell PCL-gelatin electrospun nanofibers.Bioprocess Biosyst Eng. 2020 Nov;43(11):1961-1971. doi: 10.1007/s00449-020-02385-7. Epub 2020 Jul 1. Bioprocess Biosyst Eng. 2020. PMID: 32607862
-
Poly(hydroxybutyrate)/cellulose acetate blend nanofiber scaffolds: Preparation, characterization and cytocompatibility.Mater Sci Eng C Mater Biol Appl. 2016 Jan 1;58:757-67. doi: 10.1016/j.msec.2015.09.048. Epub 2015 Sep 14. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26478369
-
Microfibers as Physiologically Relevant Platforms for Creation of 3D Cell Cultures.Macromol Biosci. 2017 Dec;17(12). doi: 10.1002/mabi.201700279. Epub 2017 Nov 17. Macromol Biosci. 2017. PMID: 29148617 Review.
References
-
- Aoki H, Miyoshi H, Yamagata Y (2015) Electrospinning of gelatin nanofiber scaffolds with mild neutral cosolvents for use in tissue engineering. Polym J 47:267–277. 10.1038/pj.2014.94
-
- Bonino CA, Krebs MD, Saquing CD, Jeong SI, Shearer KL, Alsberg E, Khan SA (2011) Electrospinning alginate-based nanofibers: from blends to crosslinked low molecular weight alginate-only systems. Carbohydr Polym 85:111–119. 10.1016/j.carbpol.2011.02.002
-
- Dawson TR, Weaver AM (2023) Niche tension controls exosome production. Nat Cell Biol 25:377–378. 10.1038/s41556-023-01088-x - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

