The response to desiccation in Acinetobacter baumannii
- PMID: 40220276
- PMCID: PMC12005421
- DOI: 10.1080/21505594.2025.2490209
The response to desiccation in Acinetobacter baumannii
Abstract
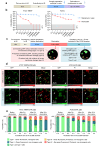
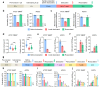
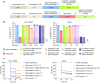
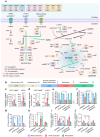
The long-term resistance to desiccation on abiotic surfaces is a key determinant of the adaptive success of Acinetobacter baumannii as a healthcare-associated bacterial pathogen. Here, the cellular and molecular mechanisms enabling A. baumannii to resist desiccation and persist on abiotic surfaces were investigated. Experiments were set up to mimic the A. baumannii response to air-drying that would occur when bacterial cells contaminate fomites in hospitals. Resistance to desiccation and transition to the "viable but nonculturable" (VBNC) state were determined in the laboratory-adapted strain ATCC 19606T and the epidemic strain ACICU. Culturability, membrane integrity, metabolic activity, virulence, and gene expression profile were compared between the two strains at different stages of desiccation. Upon desiccation, ATCC 19606T and ACICU cells lose culturability and membrane integrity, lower their metabolism, and enter the VBNC state. However, desiccated A. baumannii cells fully recover culturability and virulence in an insect infection model following rehydration in physiological buffers or human biological fluids. Transcriptome and chemical analyses of A. baumannii cells during desiccation unveiled the production of protective metabolites (L-cysteine and L-glutamate) and decreased energetic metabolism consequent to activation of the glyoxylate shunt (GS) pathway, as confirmed by reduced resuscitation efficiency of aceA mutants, lacking the key enzyme of the GS pathway. VBNC cell formation and extensive metabolic reprogramming provide a biological basis for the response of A. baumannii to desiccation, with implications on environmental control measures aimed at preventing the transmission of A. baumannii infection in hospitals.
Keywords: AceA; VBNC; glyoxylate shunt; membrane permeability; resuscitation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
