Exposure-response relationship of niraparib in maintenance therapy for recurrent ovarian cancer: ancillary analysis of the French GINECO-NiQoLe study
- PMID: 40220450
- PMCID: PMC12018549
- DOI: 10.1016/j.esmoop.2025.105054
Exposure-response relationship of niraparib in maintenance therapy for recurrent ovarian cancer: ancillary analysis of the French GINECO-NiQoLe study
Abstract
Background: Interindividual variability in pharmacokinetics may influence clinical outcomes of niraparib in patients with platinum-sensitive recurrent ovarian cancer (ROC). We aimed to investigate the pharmacokinetic-pharmacodynamic (PK-PD) relationship of niraparib in 49 patients with ROC from the multicenter phase IV NiQoLe study.
Materials and methods: Steady-state trough concentrations (Cmin,ss) on days 8 (D8) and 90 (D90) after treatment initiation were analyzed in the PK-PD analysis in regard to early dose-limiting toxicity (DLT) during the first 3 months of treatment, self-reported adverse events [Patient-Reported Outcome version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE)], and progression-free survival (PFS). Logistic regression and Cox proportional hazards models were used to identify risk factors of toxicity and predictors of PFS, respectively.
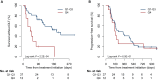
Results: The starting dose was 200 mg/day in 39 patients (80%). Nineteen patients (39%) experienced early DLT. In multivariable analysis, the fourth quartile (Q4) of Cmin,ss at D8 (≥686 ng/ml) was identified as an independent risk factor for early DLT [odds ratio (OR) 27.92, 95% confidence interval (CI) 1.99-392.53, P = 0.014] in contrast to the starting dose (OR 0.44, 95% CI 0.04-4.76, P = 0.50). High Cmin,ss at D8 was also associated with grade ≥2 self-reported adverse events, including nausea (P = 0.03) and fatigue (P = 0.02). In univariate analysis, PFS was associated neither with the 200-mg starting dose [hazard ratio (HR) 1.25, 95% CI 0.59-2.62, P = 0.57] nor with Cmin,ss at D8 (HR 1.03, 95% CI 0.90-1.19, P = 0.65). No difference in PFS was observed between Q4 and Q1-Q3 for Cmin,ss at D8 [174 days (95% CI 120 days-not reached) versus 242 days (95% CI 183-490 days), respectively; HR 1.15, 95% CI 0.57-2.36, P = 0.69]. Median PFS in patients who had a dose reduction was consistent with that of patients who remained at the starting dose [197 days (95% CI 166 days-not reached) versus 207 days (95% CI 176-469 days), respectively; log-rank P value = 0.29].
Conclusions: Increased plasma exposure at D8 (Cmin,ss ≥686 ng/ml) was associated with a higher risk of early DLT onset and patient-reported outcomes adverse events, without gain of efficacy in regard to PFS. Early plasma drug monitoring may be useful to prevent niraparib toxicity in patients with ROC treated in the maintenance phase.
Keywords: PK–PD relationship; drug monitoring; maintenance; niraparib; ovarian cancer; toxicity.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Ledermann J.A., Matias-Guiu X., Amant F., et al. ESGO–ESMO–ESP consensus conference recommendations on ovarian cancer: pathology and molecular biology and early, advanced and recurrent disease. Ann Oncol. 2024;35(3):248–266. - PubMed
-
- Mirza M.R., Monk B.J., Herrstedt J., et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. - PubMed
-
- González-Martín A., Pothuri B., Vergote I., et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–2402. - PubMed
-
- Berek J.S., Matulonis U.A., Peen U., et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29(8):1784–1792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

