Multi-kingdom gut microbiota dysbiosis is associated with the development of pulmonary arterial hypertension
- PMID: 40220715
- PMCID: PMC12013117
- DOI: 10.1016/j.ebiom.2025.105686
Multi-kingdom gut microbiota dysbiosis is associated with the development of pulmonary arterial hypertension
Abstract
Background: Gut microbiota dysbiosis has been implicated in pulmonary arterial hypertension (PAH). However, the exact roles and underlying mechanisms of multi-kingdom gut microbiota, including bacteria, archaea, and fungi, in PAH remain largely unclear.
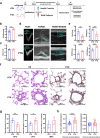
Methods: The shotgun metagenomics was used to analyse multi-kingdom gut microbial communities in patients with idiopathic PAH (IPAH) and healthy controls. Furthermore, fecal microbiota transplantation (FMT) was performed to transfer gut microbiota from IPAH patients or monocrotaline (MCT)-PAH rats to normal rats and from normal rats to MCT-PAH rats.
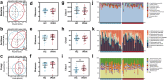
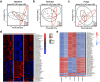
Findings: Gut microbiota analysis revealed substantial alterations in the bacterial, archaeal, and fungal communities in patients with IPAH compared with healthy controls. Notably, FMT from IPAH patients or MCT-PAH rats induced PAH phenotypes in recipient rats. More intriguingly, FMT from normal rats to MCT-PAH rats significantly ameliorated PAH symptoms; restored gut bacteria, archaea, and fungi composition; and shifted the plasma metabolite profiles of MCT-PAH rats toward those of normal rats. In parallel, RNA-sequencing analysis demonstrated the expression of genes involved in key signalling pathways related to PAH. A panel of multi-kingdom markers exhibited superior diagnostic accuracy compared with single-kingdom panels for IPAH.
Interpretation: Our findings established an association between multi-kingdom gut microbiota dysbiosis and PAH, thereby indicating the therapeutic potential of FMT in PAH. More importantly, apart from gut bacteria, gut archaea and fungi were also significantly associated with PAH pathogenesis, highlighting their indispensable role in PAH.
Funding: This work was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Projects No. 2024ZD0531200, No. 2024ZD0531201 (Research on Prevention and Treatment of Cancer, Cardiovascular and Cerebrovascular Diseases, Respiratory Diseases, and Metabolic Diseases), the National Natural Science Foundation of China of China (No. 82170302, 82370432), Financial Budgeting Project of Beijing Institute of Respiratory Medicine (Ysbz2025004, Ysbz2025007), National clinical key speciality construction project Cardiovascular Surgery, Reform and Development Program of Beijing Institute of Respiratory Medicine (Ggyfz202417, Ggyfz202501), Clinical Research Incubation Program of Beijing Chaoyang Hospital Affiliated to Capital Medical University (CYFH202209).
Keywords: Fecal microbiota transplantation; Gut microbiota; Metabolomics; Metagenomics; Pulmonary arterial hypertension.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures





Similar articles
-
Alterations in the gut mycobiome with coronary artery disease severity.EBioMedicine. 2024 May;103:105137. doi: 10.1016/j.ebiom.2024.105137. Epub 2024 May 3. EBioMedicine. 2024. PMID: 38703606 Free PMC article.
-
Unraveling the role of gut microbiota by fecal microbiota transplantation in rat model of kidney stone disease.Sci Rep. 2024 Sep 20;14(1):21924. doi: 10.1038/s41598-024-72694-4. Sci Rep. 2024. PMID: 39300177 Free PMC article.
-
The Role and Mechanism of Gut Microbiota in Pulmonary Arterial Hypertension.Nutrients. 2022 Oct 13;14(20):4278. doi: 10.3390/nu14204278. Nutrients. 2022. PMID: 36296961 Free PMC article. Review.
-
Impact of fecal microbiota transplantation on lung function and gut microbiome in an ARDS rat model: A multi-omics analysis including 16S rRNA sequencing, metabolomics, and transcriptomics.Int J Immunopathol Pharmacol. 2025 Jan-Dec;39:3946320251333982. doi: 10.1177/03946320251333982. Epub 2025 Apr 23. Int J Immunopathol Pharmacol. 2025. PMID: 40265594 Free PMC article.
-
Role of Gut Microbiota in Pulmonary Arterial Hypertension.Front Cell Infect Microbiol. 2022 May 6;12:812303. doi: 10.3389/fcimb.2022.812303. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35601107 Free PMC article. Review.
Cited by
-
Respiratory diseases and the gut microbiota: an updated review.Front Cell Infect Microbiol. 2025 Aug 11;15:1629005. doi: 10.3389/fcimb.2025.1629005. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40861492 Free PMC article. Review.
References
-
- Hoeper M.M., Pausch C., Grunig E., et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transpl. 2020;39:1435–1444. - PubMed
-
- Yan Q., Li S., Yan Q., et al. A genomic compendium of cultivated human gut fungi characterizes the gut mycobiome and its relevance to common diseases. Cell. 2024;187:2969–2989. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

