Human MAPT knockin mouse models of frontotemporal dementia for the neurodegenerative research community
- PMID: 40220760
- PMCID: PMC12256944
- DOI: 10.1016/j.crmeth.2025.101024
Human MAPT knockin mouse models of frontotemporal dementia for the neurodegenerative research community
Abstract
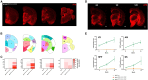
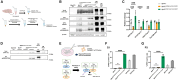
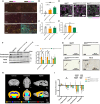
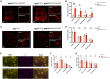
Existing models of frontotemporal dementia (FTD) may not fully recapitulate the pathophysiology of the disease. To generate more pathophysiologically relevant FTD models, we engineered MAPT knockin mouse lines carrying triple mutations, among which the MAPTP301S;Int10+3;S320F line exhibited robust tau pathology starting before 6 months of age. Severe tau accumulation was predominantly observed in the thalamus, hypothalamus, and amygdala with milder involvement of the cortex and hippocampus, leading to synaptic loss, brain atrophy, and FTD-like behavioral abnormalities. Crossbreeding MAPTP301S;Int10+3;S320F mice with App knockin, AppNL-G-F, mice markedly enhanced tau pathology in the cortex and hippocampus, highlighting the interplay between β-amyloid and tau. These findings establish the mutant mice as valuable models for investigating the mechanisms underlying FTD and other tauopathies, providing a relevant platform for in vivo drug screening.
Keywords: CP: neuroscience; IntelliCage; MAPT knockin mouse; frontotemporal dementia; genome editing; tauopathies.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The RIKEN IP Office has filed patents for the triple mutant MAPT KI mouse lines with N.W., T.M., N.K., M.Q., H.S., and T.C.S. as inventors. T.C.S. serves as a consultant for RIKEN BIO Co. Ltd.
Figures




References
-
- Saito T., Mihira N., Matsuba Y., Sasaguri H., Hashimoto S., Narasimhan S., Zhang B., Murayama S., Higuchi M., Lee V.M.Y., et al. Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J. Biol. Chem. 2019;294:12754–12765. doi: 10.1074/jbc.RA119.009487. - DOI - PMC - PubMed
-
- Watamura N., Foiani M., Bez S., Bourdenx M., Santambrogio A., Frodsham C., Camporesi E., Brinkmalm G., Zetterberg H., Patel S., et al. In vivo hyperphosphorylation of tau is associated with synaptic loss and behavioral abnormalities in the absence of tau seeds. Nat. Neurosci. 2024:1–15. in press. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

