Gasdermin D deficiency aggravates nephrocalcinosis-related chronic kidney disease with rendering macrophages vulnerable to necroptosis
- PMID: 40221396
- PMCID: PMC11993636
- DOI: 10.1038/s41419-025-07620-1
Gasdermin D deficiency aggravates nephrocalcinosis-related chronic kidney disease with rendering macrophages vulnerable to necroptosis
Abstract
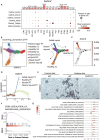
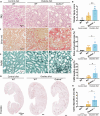
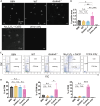
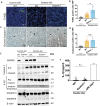
Several forms of regulated necrosis contribute to the pathogenesis of crystal nephropathy, however, the role of pyroptosis, an inflammatory form of cell death involving the formation of gasdermin-D pores in internal and external cell membranes, in this condition remains unknown. Our transcriptional and histological analyses suggest that Gsdmd in tubulointerstitital cells may contribute to the pathogenesis of chronic oxalate nephropathy. However, genetic deletion of Gsdmd exacerbated oxalate nephropathy in mice in association with enhanced CaOx crystal deposition and accelerated tubular epithelial cell injury. Pharmacological inhibition of necroptosis reversed this effect. Indeed, Gsdmd-/- bone marrow-derived macrophages were more prone to undergo necroptosis when stimulated with CaOx crystals compared to their wildtype counterparts. We conclude that gasdermin D suppresses the necroptosis pathway, which determines the outcome of oxalate nephropathy-related nephrocalcinosis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All animal experimental procedures were performed in accordance with the European law regarding protection of animal welfare (directive 2010/63/EU). The use of animals for this study was approved by the local government authorities, Regierung von Oberbayern (approval code ROB-55.2Vet-2532.Vet_02-20-101). Open Access funding enabled and organized by Projekt DEAL
Figures



Similar articles
-
Regulating the balance between GSDMD-mediated pyroptosis and CHMP4B-dependent cell repair attenuates calcium oxalate kidney stone formation.Int J Biol Sci. 2025 Apr 22;21(7):3099-3121. doi: 10.7150/ijbs.105415. eCollection 2025. Int J Biol Sci. 2025. PMID: 40384863 Free PMC article.
-
RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis.Cell Death Differ. 2020 Sep;27(9):2568-2585. doi: 10.1038/s41418-020-0524-1. Epub 2020 Mar 9. Cell Death Differ. 2020. PMID: 32152555 Free PMC article.
-
GSDMD and GSDME synergy in the transition of acute kidney injury to chronic kidney disease.Nephrol Dial Transplant. 2024 Jul 31;39(8):1344-1359. doi: 10.1093/ndt/gfae014. Nephrol Dial Transplant. 2024. PMID: 38244230
-
Ferroptosis, necroptosis, and pyroptosis in calcium oxalate crystal-induced kidney injury.Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167791. doi: 10.1016/j.bbadis.2025.167791. Epub 2025 Mar 13. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40086520 Review.
-
GSDMD in regulated cell death: A novel therapeutic target for sepsis.Int Immunopharmacol. 2024 Jun 30;135:112321. doi: 10.1016/j.intimp.2024.112321. Epub 2024 May 24. Int Immunopharmacol. 2024. PMID: 38795599 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

