Loss of the APP regulator RHBDL4 preserves memory in an Alzheimer's disease mouse model
- PMID: 40221411
- PMCID: PMC11993729
- DOI: 10.1038/s41419-025-07579-z
Loss of the APP regulator RHBDL4 preserves memory in an Alzheimer's disease mouse model
Abstract
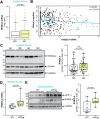
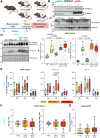
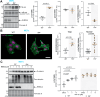
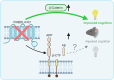
Characteristic cerebral pathological changes of Alzheimer's disease (AD) such as glucose hypometabolism or the accumulation of cleavage products of the amyloid precursor protein (APP), known as Aβ peptides, lead to sustained endoplasmic reticulum (ER) stress and neurodegeneration. To preserve ER homeostasis, cells activate their unfolded protein response (UPR). The rhomboid-like-protease 4 (RHBDL4) is an enzyme that participates in the UPR by targeting proteins for proteasomal degradation. We demonstrated previously that RHBDL4 cleaves APP in HEK293T cells, leading to decreased total APP and Aβ. More recently, we showed that RHBDL4 processes APP in mouse primary mixed cortical cultures as well. Here, we aim to examine the physiological relevance of RHBDL4 in the brain. We first found that brain samples from AD patients and an AD mouse model (APPtg) showed increased RHBDL4 mRNA and protein expression. To determine the effects of RHBDL4's absence on APP physiology in vivo, we crossed APPtg mice to a RHBDL4 knockout (R4-/-) model. RHBDL4 deficiency in APPtg mice led to increased total cerebral APP and amyloidogenic processing when compared to APPtg controls. Contrary to expectations, as assessed by cognitive tests, RHBDL4 absence rescued cognition in 5-month-old female APPtg mice. Informed by unbiased RNA-seq data, we demonstrated in vitro and in vivo that RHBDL4 absence leads to greater levels of active β-catenin due to decreased proteasomal clearance. Decreased β-catenin activity is known to underlie cognitive defects in APPtg mice and AD. Our work suggests that RHBDL4's increased expression in AD, in addition to regulating APP levels, leads to aberrant degradation of β-catenin, contributing to cognitive impairment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was conducted in compliance with relevant guidelines and regulations. All animal procedures were approved by McGill’s Animal Care Committee (Animal Use Protocol # MCGL-7868) and performed in accordance with the ARRIVE guidelines. The human data used in this study were obtained from the Religious Orders Study and the ROSMAP study, approved by the Institutional Review Board of Rush University Medical Center, as well as from the Center for Brain Aging & Dementia Tissue Repository at University of California, Irvine. Each participant provided informed consent, including an Anatomic Gift Act and repository consent, to allow their data to be repurposed for research.
Figures



Update of
-
Loss of the APP regulator RHBDL4 preserves memory in an Alzheimer's disease mouse model.bioRxiv [Preprint]. 2024 Sep 8:2024.02.22.579698. doi: 10.1101/2024.02.22.579698. bioRxiv. 2024. Update in: Cell Death Dis. 2025 Apr 12;16(1):280. doi: 10.1038/s41419-025-07579-z. PMID: 38464180 Free PMC article. Updated. Preprint.
Similar articles
-
Loss of the APP regulator RHBDL4 preserves memory in an Alzheimer's disease mouse model.bioRxiv [Preprint]. 2024 Sep 8:2024.02.22.579698. doi: 10.1101/2024.02.22.579698. bioRxiv. 2024. Update in: Cell Death Dis. 2025 Apr 12;16(1):280. doi: 10.1038/s41419-025-07579-z. PMID: 38464180 Free PMC article. Updated. Preprint.
-
Alternative Processing of the Amyloid Precursor Protein Family by Rhomboid Protease RHBDL4.J Biol Chem. 2016 Oct 14;291(42):21903-21912. doi: 10.1074/jbc.M116.753582. Epub 2016 Aug 25. J Biol Chem. 2016. PMID: 27563067 Free PMC article.
-
Eta-secretase-like processing of the amyloid precursor protein (APP) by the rhomboid protease RHBDL4.J Biol Chem. 2024 Aug;300(8):107541. doi: 10.1016/j.jbc.2024.107541. Epub 2024 Jul 9. J Biol Chem. 2024. PMID: 38992438 Free PMC article.
-
ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer's Neuronal Pathology.J Neurosci. 2016 Mar 30;36(13):3848-59. doi: 10.1523/JNEUROSCI.3757-15.2016. J Neurosci. 2016. PMID: 27030769 Free PMC article.
-
Understanding the function of ABCA7 in Alzheimer's disease.Biochem Soc Trans. 2015 Oct;43(5):920-3. doi: 10.1042/BST20150105. Biochem Soc Trans. 2015. PMID: 26517904 Review.
Cited by
-
Brain Endothelial Cells in Blood-Brain Barrier Regulation and Neurological Therapy.Int J Mol Sci. 2025 Jun 18;26(12):5843. doi: 10.3390/ijms26125843. Int J Mol Sci. 2025. PMID: 40565303 Free PMC article. Review.
References
-
- Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9:25–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

