Cortico force coherence of the finger and toe with slight rhythmic pressure on force sensors using electroencephalography
- PMID: 40221443
- PMCID: PMC11993576
- DOI: 10.1038/s41598-025-95759-4
Cortico force coherence of the finger and toe with slight rhythmic pressure on force sensors using electroencephalography
Abstract
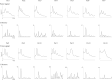
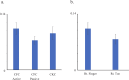
We investigated the usefulness of cortico-force coherence (CFC) between electroencephalography (EEG) and force signals for assessing sensorimotor cortex function. Fourteen healthy participants performed slight rhythmical pressing with the right finger and right toe at a self-paced rate of 1-3 Hz under the active condition of CFC using a force sensor and electromyography (EMG). For passive CFC, the experimenter pressed the participant's right finger at a rate similar to that in the active condition. As control, the conventional corticokinematic coherence (CKC) was recorded for the right finger using an accelerometer (ACC). We also recorded CFC in the active condition by pressing the right toe. In all participants, coherence spectra between the 32-channel EEG signals and force, ACC, and EMG signals showed significant peaks (P < 0.01) at the movement frequency peaks or their harmonics. Finger CFC peak value did not differ among the three conditions (active CFC, passive CFC, and CKC). Finger CFC, force sensor, and EMG values showed no differences. Additionally, finger CFC did not significantly differ from toe CFC. The CFC approach with EEG appears promising and useful for the functional assessment of the sensorimotor cortex, with a clinical advantage of conducting measurements using less force and without obvious kinematics.
Keywords: Corticokinematic coherence; Electroencephalography; Motor-evoked fields; Primary sensorimotor cortex; Sophisticated movements; Voluntary movement.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

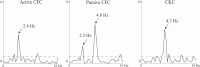

Similar articles
-
MEG-compatible pneumatic stimulator to elicit passive finger and toe movements.Neuroimage. 2015 May 15;112:310-317. doi: 10.1016/j.neuroimage.2015.03.006. Epub 2015 Mar 11. Neuroimage. 2015. PMID: 25770989
-
Corticokinematic coherence during active and passive finger movements.Neuroscience. 2013 May 15;238:361-70. doi: 10.1016/j.neuroscience.2013.02.002. Epub 2013 Feb 10. Neuroscience. 2013. PMID: 23402851
-
Functional cortical localization of tongue movements using corticokinematic coherence with a deep learning-assisted motion capture system.Sci Rep. 2022 Jan 10;12(1):388. doi: 10.1038/s41598-021-04469-0. Sci Rep. 2022. PMID: 35013521 Free PMC article.
-
Feasibility and reproducibility of electroencephalography-based corticokinematic coherence.J Neurophysiol. 2020 Dec 1;124(6):1959-1967. doi: 10.1152/jn.00562.2020. Epub 2020 Oct 28. J Neurophysiol. 2020. PMID: 33112711
-
Coupling between human brain activity and body movements: Insights from non-invasive electromagnetic recordings.Neuroimage. 2019 Dec;203:116177. doi: 10.1016/j.neuroimage.2019.116177. Epub 2019 Sep 9. Neuroimage. 2019. PMID: 31513941 Review.
References
-
- Bourguignon, M. et al. Functional motor-cortex mapping using corticokinematic coherence. Neuroimage55, 1475–1479 (2011). - PubMed
-
- Bourguignon, M. et al. Neuronal network coherent with hand kinematics during fast repetitive hand movements. Neuroimage59, 1684–1691 (2012). - PubMed
-
- Piitulainen, H., Bourguignon, M., De Tiège, X., Hari, R. & Jousmäki, V. Corticokinematic coherence during active and passive finger movements. Neuroscience238, 361–370 (2013). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

