Propensity score analysis of high-dose rate brachytherapy, immune checkpoint inhibitors, and docetaxel in second-line advanced NSCLC treatment
- PMID: 40221605
- PMCID: PMC11993689
- DOI: 10.1038/s41598-025-97918-z
Propensity score analysis of high-dose rate brachytherapy, immune checkpoint inhibitors, and docetaxel in second-line advanced NSCLC treatment
Abstract
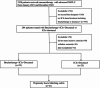
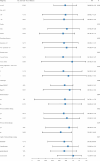
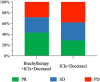
This study evaluated the efficacy and safety of combining high-dose-rate brachytherapy, immune checkpoint inhibitors, and docetaxel as second-line treatment for advanced NSCLC, given the poor prognosis after first-line therapy. We conducted a single-center, retrospective, propensity score-matched study comparing HDR brachytherapy plus ICIs and docetaxel (study group) versus ICIs plus docetaxel (control group) in patients with advanced NSCLC who progressed after prior treatment without known driver gene mutations or uninvestigated mutation status. After propensity score matching, 21 patients were included in each group. The study group had a higher ORR (42.9% vs. 28.6%). Median OS was 18.6 months for the study group and 12.8 months for the control group (HR 0.45, 95% CI 0.20-0.85, P = 0.042). Median PFS was 8.6 vs. 5.6 months (HR 0.29, 95% CI 0.15-0.55, P < 0.001). The DCR was higher in the study group (71.4% vs. 61.9%). Treatment-related AEs were manageable, with no significant increase in grade 3/4 toxicities in the study group. Results suggest that combining high-dose rate brachytherapy, immune checkpoint inhibitors, and docetaxel may improve survival and response rates in advanced NSCLC after first-line therapy. Prospective randomized trials are necessary to confirm these findings and validate the strategy's effectiveness.
Keywords: High-dose-rate brachytherapy; Immune checkpoint inhibitors; Non-small cell lung cancer; Propensity score matching; Treatment-related adverse events.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: The study was approved by the Human Research Ethics Committees of The Second People’s Hospital of Neijiang in accordance with the Declaration of Helsinki. Written informed consent was obtained from all individual patients included in the study.
Figures


Similar articles
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Analysis of ICIs alone or in combination rechallenged outcomes after progression from first-line ICIs plus chemotherapy in patients with advanced non-small cell lung cancer.Sci Rep. 2025 Jan 2;15(1):30. doi: 10.1038/s41598-024-83947-7. Sci Rep. 2025. PMID: 39747923 Free PMC article.
-
[A Comparative Study of the Efficacy and Safety of Immune Monotherapy versus Immunotheray Combined with Chemotherapy in Elderly Patients Aged 75 Years and Above with Advanced Non-small Cell Lung Cancer].Zhongguo Fei Ai Za Zhi. 2024 Sep 20;27(9):665-673. doi: 10.3779/j.issn.1009-3419.2024.101.21. Zhongguo Fei Ai Za Zhi. 2024. PMID: 39492581 Free PMC article. Chinese.
-
Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial.JAMA Oncol. 2020 Jun 1;6(6):856-864. doi: 10.1001/jamaoncol.2020.0409. JAMA Oncol. 2020. PMID: 32271354 Free PMC article. Clinical Trial.
References
-
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. 10.3322/caac.21660 (2021). - PubMed
-
- Xu, S. et al. Exploring FNDC4 as a biomarker for prognosis and immunotherapy response in lung adenocarcinoma. Asian J. Surg.10.1016/j.asjsur.2024.09.054 (2024). - PubMed
-
- Xu, S. et al. The role of LMNB2 as a diagnostic and prognostic biomarker in lung adenocarcinoma. Asian J. Surg.10.1016/j.asjsur.2024.08.056 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

