Implementation outcomes of a patient decision-aid in a diverse population with systemic lupus erythematosus in 15 US rheumatology clinics
- PMID: 40221856
- PMCID: PMC12316375
- DOI: 10.1093/rheumatology/keaf205
Implementation outcomes of a patient decision-aid in a diverse population with systemic lupus erythematosus in 15 US rheumatology clinics
Abstract
Objective: The objective of this study was to examine the clinical outcomes during the implementation of a self-administered patient decision-aid (PtDA) for lupus.
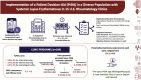
Methods: We provided an effective computerized lupus PtDA in 15 rheumatology outpatient clinics 2019-2024 (including the COVID pandemic). We undertook Organizational Readiness to Implement Change Surveys (ORICs) and Team Learning and Psychological Safety Surveys (TLPSSs) at baseline. The primary study outcome objective measure, percent penetration/reach, was defined as the number of patients who viewed the lupus PtDA at the end of the study, divided by the total number of eligible patients (times 100). We used validated clinical personnel surveys to examine the perceived lupus PtDA appropriateness, acceptability, feasibility, success, and permanence, at 4 months, 12 months and 24 months post-PtDA implementation.
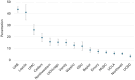
Results: Among the sites, the clinical personnels' (n = 184) baseline ORIC commitment and efficacy scores (a 0-5 scale, higher = better) ranged from 3.5 to 4.2, and 3.4 to 4.4, respectively; the TLPSS scores (a 0-7 scale, higher = better) were 3.9-5.5 for internal learning, 3.7-5.6 for external learning, and 4.3-5.5 for psychological safety. The penetration (primary outcome) among 15 geographically diverse US rheumatology clinics ranged from 3% to 44%. We found that the total number of providers in the clinic was positively associated with higher penetration. Clinical personnel-perceived lupus PtDA outcomes were optimal at 4 months (all scale scores ranged from 1 to 5, higher = better): (i) appropriateness, 3.43 (s.d. 0.86); (ii) acceptability, 3.53 (s.d. 0.83); (iii) feasibility, 3.44 (s.d. 0.71); (iv) success, 3.41 (s.d. 0.73); and (v) permanence, 3.22 (s.d. 0.74).
Conclusion: We implemented a lupus PtDA with varied success rates during the COVID pandemic; more providers were associated with higher clinic penetration. Clinical personnel perceived high lupus PtDA appropriateness, acceptability, feasibility, success, and permanence that persisted up to 24 months.
Trial registration: ClinicalTrials.gov, http://clinicaltrials.gov, NCT03735238.
Keywords: clinical outcomes; clinical personnel; decision-aid; education; health-care delivery; implementation; rheumatology clinic; systemic lupus erythematosus.
Published by Oxford University Press on behalf of the British Society for Rheumatology 2025.
Figures
Similar articles
-
Organizational Climate and Decision Aid Sustainability in Lupus Care: Mixed Methods Study.JMIR Form Res. 2025 Aug 21;9:e69603. doi: 10.2196/69603. JMIR Form Res. 2025. PMID: 40838956 Free PMC article.
-
Where, how, and how much? A multicenter cohort study of the relationship between lupus decision aid modality, place of administration, interruption and viewing completeness and patient-reported outcomes.Arthritis Care Res (Hoboken). 2025 Jul 14. doi: 10.1002/acr.25603. Online ahead of print. Arthritis Care Res (Hoboken). 2025. PMID: 40654136
-
How Does Shame Relate to Clinical and Psychosocial Outcomes in Knee Osteoarthritis?Clin Orthop Relat Res. 2025 Apr 1;483(4):558-570. doi: 10.1097/CORR.0000000000003329. Epub 2024 Dec 3. Clin Orthop Relat Res. 2025. PMID: 39625176
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Barr RG, Seliger S, Appel GB et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003;18:2039–46. - PubMed
-
- Bernatsky S, Boivin JF, Joseph L et al. Race/ethnicity and cancer occurrence in systemic lupus erythematosus. Arthritis Rheum 2005;53:781–4. - PubMed
-
- Helmick CG, Felson DT, Lawrence RC et al. ; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008;58:15–25. - PubMed
-
- Maisonneuve P, Agodoa L, Gellert R et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis 2000;35:157–65. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- R01 AR080089/GF/NIH HHS/United States
- R01 AI169671/AI/NIAID NIH HHS/United States
- U18 HS027946/HS/AHRQ HHS/United States
- NIH
- R01AI169671/National Institute for Allergy and Infectious Diseases
- R01 AI170938/AI/NIAID NIH HHS/United States
- Lupus Foundation of America
- P30 AR072582/AR/NIAMS NIH HHS/United States
- VA Medical Center at Houston
- U18HS027946/HS/AHRQ HHS/United States
- P30 AR072579/AR/NIAMS NIH HHS/United States
- R01MD015395/Medical University of South Carolina
- P50 MD017338/MD/NIMHD NIH HHS/United States
- NU27DD000022/Centers for Disease Control
- R01AI155052/National Institute for Allergy and Infectious Diseases
- P30 AI027767/AI/NIAID NIH HHS/United States
- U01AR071077/Medical University of South Carolina
- P30AI027767/National Institute for Allergy and Infectious Diseases
- U01AI176135/Medical University of South Carolina
- R01 CA271303/CA/NCI NIH HHS/United States
- UM1AI109565/Medical University of South Carolina
- U01AI125159/Medical University of South Carolina
- UM1 AI109565/AI/NIAID NIH HHS/United States
- R01 AI170938/GF/NIH HHS/United States
- R21 AR084039/AR/NIAMS NIH HHS/United States
- R21AR084039/Medical University of South Carolina
- R01 MD015395/MD/NIMHD NIH HHS/United States
- R01 AI155052/AI/NIAID NIH HHS/United States
- U01 AI184159/AI/NIAID NIH HHS/United States
- P30 AR073752/AR/NIAMS NIH HHS/United States
- U01 AI125159/AI/NIAID NIH HHS/United States
- UM1 TR004771/TR/NCATS NIH HHS/United States
- U01 AR071077/AR/NIAMS NIH HHS/United States
- R21 AI171491/AI/NIAID NIH HHS/United States
- R21AI171491/GF/NIH HHS/United States
- SDM-2017C2-8224/PCORI/Patient-Centered Outcomes Research Institute/United States
- R01 AR071091/AR/NIAMS NIH HHS/United States
- R01 AR080089/AR/NIAMS NIH HHS/United States
- P30AR072582/Medical University of South Carolina
- U01AI184159/Medical University of South Carolina
- P50MD017338/National Institute for Minority Health and Disparities
- R01AR071091/GF/NIH HHS/United States
- UM1TR004771/National Center for Advancing Translational Science
- U01 AI176135/AI/NIAID NIH HHS/United States
- CER-2020C1-19193/PI/PCORI HHS/United States
- P30AR072579/GF/NIH HHS/United States
- P50 AR060772/AR/NIAMS NIH HHS/United States
- R01CA271303/National Institute for Minority Health and Disparities
LinkOut - more resources
Full Text Sources
Medical

