C-terminal frameshift variants in GPKOW are associated with a multisystemic X-linked disorder
- PMID: 40221893
- PMCID: PMC12435177
- DOI: 10.1016/j.gim.2025.101429
C-terminal frameshift variants in GPKOW are associated with a multisystemic X-linked disorder
Abstract
Purpose: GPKOW, a gene on the X-chromosome, encodes a nuclear RNA-binding protein important in messenger RNA (mRNA) processing as a spliceosome subunit. This work aims to establish GPKOW as a disease-associated gene.
Methods: We describe 3 males from 2 unrelated families with hemizygous frameshift variants affecting the last exon of GPKOW p.(Arg441SerfsTer30) and p.(Ser444GlufsTer28). The effect of p.(Ser444GlufsTer28) on gene expression was evaluated in patient's fibroblasts. In vivo studies in Drosophila melanogaster targeting the sole GPKOW fly ortholog, CG10324 (Gpkow) were performed.
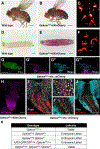
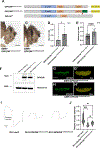
Results: Clinical presentations included intrauterine growth restriction, microcephaly/microencephaly, and eye, brain, skin, and skeletal abnormalities. Heterozygote females presented with short stature, microcephaly, and vision problems. Sequencing of fibroblasts' mRNA confirmed that GPKOW mRNA escapes nonsense-mediated decay. Yet, reduced protein levels suggested protein instability. Studies in Drosophila showed that Gpkow is essential and broadly expressed. It is enriched in neurons and glia in eyes and head of developing and adult flies. Knockdown and overexpression of Gpkow in the fly eye cause eyeless/headless phenotype, suggesting that the gene is dosage sensitive. Importantly, overexpression of the p.(Ser444GlufsTer28) variant caused milder defects than the reference allele, indicating that the truncated protein behaves as a partial loss-of-function allele.
Conclusion: Rare variants in GPKOW cause a multisystemic X-linked syndrome.
Keywords: CG10324; Drosophila melanogaster; GPKOW; Minor Spliceosome; Neurodevelopment.
Copyright © 2025 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare no conflicts of interest.
Figures


References
-
- Hegele A, Kamburov A, Grossmann A, et al. Dynamic protein-protein interaction wiring of the human spliceosome. Mol Cell. 2012;45(4):567–580. - PubMed
-
- Piano F, Schetter AJ, Morton DG, et al. Gene clustering based on RNAi phenotypes of ovary-enriched genes in C. elegans. Curr Biol. 2002;12(22):1959–1964. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

