A polytherapy approach demonstrates therapeutic efficacy for the treatment of SOD1 associated amyotrophic lateral sclerosis
- PMID: 40222103
- PMCID: PMC12018197
- DOI: 10.1016/j.ebiom.2025.105692
A polytherapy approach demonstrates therapeutic efficacy for the treatment of SOD1 associated amyotrophic lateral sclerosis
Abstract
Background: SOD1 mutations are a significant contributor of familial amyotrophic lateral sclerosis (ALS) cases. SOD1 mutations increase the propensity for the protein to misfold and aggregate into insoluble proteinaceous deposits within motor neurons and neighbouring cells. The small molecule, CuATSM, has repeatedly shown in mouse models to be a promising therapeutic treatment for SOD1-associated ALS and is currently in Phase II/III clinical trials for the treatment of ALS. We have previously shown CuATSM stabilises various ALS-associated variants of the SOD1 protein, reducing misfolding and toxicity. Two additional FDA-approved small molecules, ebselen and telbivudine, have also been identified to reduce mutant SOD1 toxicity, providing additional potential therapeutic candidates that could be used in combination with CuATSM. Here, we aimed to investigate if CuATSM, ebselen and telbivudine (CET) polytherapy could improve on the therapeutic efficacy of CuATSM monotherapy for the treatment of SOD1-associated ALS.
Methods: We utilised a 3D checkerboard approach to investigate whether a matrix of different concentrations CuATSM, ebselen and telbivudine could provide therapeutic improvements on cell survival, SOD1 folding and aggregation in SOD1G93A-transfected NSC-34 cells, compared to CuATSM alone. To progress the preclinical development of CET polytherapy, we evaluated the bioavailability and safety of in vivo polytherapy administration. Furthermore, we assessed and compared the effects of CET- and CuATSM-treatment on disease onset, motor function, survival and neuropathological features in SOD1G93A mice.
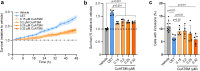
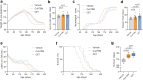
Findings: CET polytherapy reduced inclusion formation and increased cell survival of NSC-34 cells overexpressing SOD1G93A compared to higher concentrations of CuATSM monotherapy. In addition, CET administration was bioavailable and tolerable in mice. CET treatment in SOD1G93A mice delayed disease onset, reduced motor impairments, and increased survival compared to vehicle- and CuATSM-treated mice. In line with these findings, biochemical analysis of lumbar spinal cords showed CET administration improved SOD1 folding, decreased misfolded SOD1 accumulation, and reduced motor neuron loss.
Interpretation: These findings support CET polytherapy as an advantageous alternative compared to CuATSM monotherapy and highlight the potential of utilising small molecules targeting SOD1 as a polytherapy avenue for the treatment of SOD1-associated ALS.
Funding: This work was supported by a FightMND Drug Development Grant, an Australian National Health and Medical Research Council (NHMRC) Investigator Grant (No. 1194872) and a Motor Neuron Disease Research Institute of Australia Bill Gole Postdoctoral Fellowship.
Keywords: Amyotrophic lateral sclerosis; CuATSM; Ebselen; Motor neuron disease; Polytherapy; SOD1; Telbivudine.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests P.S.D is named as inventor on intellectual property that relates to this research has been licenced from the University of Melbourne to Collaborative Medicinal Development. Collaborative Medicinal Development licenced intellectual property pertaining to CuATSM from the University of Melbourne where PJC is an employee but not a beneficiary of the licence agreement. P.J.C is an unpaid consultant for Collaborative Medicinal Development LLC. J.S.L received grant funding from Molecular Horizons and the University of Wollongong in the form of a Collaboration Grant (M2024). M.L.B, N.E.F, R.B, A.D, F.D, C.G.C, J.G, L.E.M, L.M and J.J.Y declare no conflicts of interest.
Figures




References
-
- Abel O., Powell J.F., Andersen P.M., Al-Chalabi A. ALSoD: a user-friendly online bioinformatics tool for amyotrophic lateral sclerosis genetics. Hum Mutat. 2012;33(9):1345–1351. - PubMed
-
- Casareno R.L., Waggoner D., Gitlin J.D. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J Biol Chem. 1998;273(37):23625–23628. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

