A replicating RNA vaccine confers protection against Crimean-Congo hemorrhagic fever in cynomolgus macaques
- PMID: 40222105
- PMCID: PMC12018191
- DOI: 10.1016/j.ebiom.2025.105698
A replicating RNA vaccine confers protection against Crimean-Congo hemorrhagic fever in cynomolgus macaques
Abstract
Background: Crimean-Congo hemorrhagic fever is a tick-borne febrile illness with wide geographic distribution. In recent years the geographic range of CCHFV and its tick vector have increased, placing an increasing number of people at risk of CCHFV infection. Currently there are no widely available vaccines and although the World Health Organization recommends ribavirin for treatment, its efficacy is unclear. Vaccines are critically needed for CCHFV.
Methods: Here we evaluated a promising replicating RNA vaccine for CCHFV in a Cynomolgus macaque model of disease.
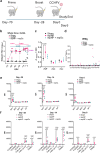
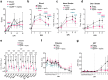
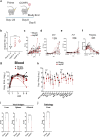
Findings: In primed and boosted macaques, we found that our replicating RNA vaccine expressing the CCHFV nucleoprotein (repNP) was highly immunogenic, eliciting a robust non-neutralizing antibody response that conferred significant protection against CCHFV challenge. Macaques receiving a single repNP vaccination were partially protected against CCHFV challenge.
Interpretation: Our data demonstrate that our repNP vaccine and NP-specific antibody can protect against CCHFV in non-human primates.
Funding: This study was supported by the Intramural Research Program of the NIAID/NIH and the Medical CBRN Defense Consortium grant #MCDC2204-011.
Keywords: Antibody; Crimean-Congo; Non-human primates; RNA vaccine; Vaccine.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of interests J.E. and A.K. have equity interest in HDT Bio. J.E. and A.K. are co-inventors on U.S. patent application no. 62/993,307 “Compositions and methods for delivery of RNA” pertaining to formulations for RNA delivery. DWH, JE and HF are inventors on U.S. patent application number 63/365,015 “Replicating RNA vaccine for Crimean-Congo hemorrhagic fever virus” regarding the repRNA for use against CCHFV. WG, HB, LM have equity interest in Seromyx systems.
Figures

References
-
- Hawman D.W., Feldmann H. Crimean-Congo haemorrhagic fever virus. Nat Rev Microbiol. 2023;21(7):463–477. https://www.ncbi.nlm.nih.gov/pubmed/36918725 Available from: - PMC - PubMed
-
- Leventhal S.S., Meade-White K., Rao D., et al. Replicating RNA vaccination elicits an unexpected immune response that efficiently protects mice against lethal Crimean-Congo hemorrhagic fever virus challenge. eBioMedicine. 2022;82 https://www.sciencedirect.com/science/article/pii/S2352396422003693 Available from: - PMC - PubMed
-
- Leventhal S.S., Meade-White K., Shaia C., et al. Single dose, dual antigen RNA vaccines protect against lethal Crimean-Congo haemorrhagic fever virus infection in mice. eBioMedicine. 2024;101 https://www.sciencedirect.com/science/article/pii/S2352396424000525 Available from: - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

