Regulation of ferryl reactivity by the cytochrome P450 decarboxylase OleT
- PMID: 40222261
- PMCID: PMC12210280
- DOI: 10.1016/j.jinorgbio.2025.112912
Regulation of ferryl reactivity by the cytochrome P450 decarboxylase OleT
Abstract
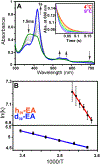
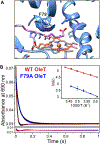
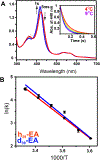
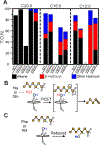
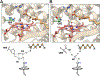
The cytochrome P450 OleT catalyzes the decarboxylation of long-chain fatty acid substrates to produce terminal alkenes using hydrogen peroxide as a co-substrate. The facile activation of peroxide to form Compound I in the first step of the reaction, and subsequent CC bond cleavage mediated by Compound II, provides a unique opportunity to visualize both ferryl intermediates using transient kinetic approaches. Analysis of the Arrhenius behavior yields activation barriers of ∼6 kcal/mol and ∼ 18 kcal/mol for the decay of Compound I and Compound II respectively. The influence of the secondary coordination sphere, probed through site-directed mutagenesis approaches, suggests that restriction of the donor-acceptor distance contributes to the reactivity of Compound I. The reactivity of Compound II was further probed using kinetic solvent isotope effect approaches, confirming that the large barrier owes to a proton-gated mechanism in the decarboxylation reaction coordinate. Hydrogen-bonding to an active-site histidine (H85) in the distal pocket plays a key role in this process.
Keywords: CYP152; Compound I; Cytochrome P450.
Copyright © 2025 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Thomas M. Makris reports financial support was provided by National Science Foundation. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
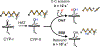
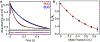
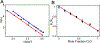
Similar articles
-
Unique structural features define the decarboxylation activity of a CYP152 fatty acid decarboxylase from Lacicoccus alkaliphilus.J Biol Chem. 2025 Jul;301(7):110397. doi: 10.1016/j.jbc.2025.110397. Epub 2025 Jun 19. J Biol Chem. 2025. PMID: 40543591 Free PMC article.
-
The Enigmatic P450 Decarboxylase OleT Is Capable of, but Evolved To Frustrate, Oxygen Rebound Chemistry.Biochemistry. 2017 Jul 5;56(26):3347-3357. doi: 10.1021/acs.biochem.7b00338. Epub 2017 Jun 26. Biochemistry. 2017. PMID: 28603981 Free PMC article.
-
Cytochrome P450BM-3 and P450 11A1 retain Compound I (FeO3+) chemistry with electrophilic substrates poised for Compound 0 (Fe3+O2-) reactions.J Biol Chem. 2025 Jul;301(7):110378. doi: 10.1016/j.jbc.2025.110378. Epub 2025 Jun 14. J Biol Chem. 2025. PMID: 40523616 Free PMC article.
-
Nicotine receptor partial agonists for smoking cessation.Cochrane Database Syst Rev. 2023 May 5;5(5):CD006103. doi: 10.1002/14651858.CD006103.pub8. Cochrane Database Syst Rev. 2023. PMID: 37142273 Free PMC article.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4. PMID: 28752910 Free PMC article. Updated.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

