Exploiting FcRn engagement of an albumin-CpG oligonucleotide covalent conjugate for potent TLR9 immune induction
- PMID: 40222546
- PMCID: PMC12148431
- DOI: 10.1016/j.jbc.2025.108508
Exploiting FcRn engagement of an albumin-CpG oligonucleotide covalent conjugate for potent TLR9 immune induction
Abstract
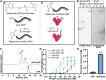
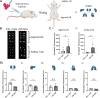
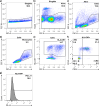
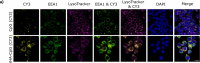
CpG-oligodeoxynucleotide (CpG ODN)-based Toll-like receptor (TLR) agonists are promising immunostimulatory adjuvants; however, low entry into TLR-rich cellular endosomal compartments and poor lymphatic accumulation limit clinical translation. In this work, we introduce a recombinant human serum albumin (rHA)-CpG ODN covalent conjugate (rHA-CpG) designed to exploit the neonatal Fc receptor (FcRn)-driven albumin cellular sorting pathway to maximize CpG delivery into TLR9-rich endosomes and accumulate in lymph nodes. Site-selective conjugation of CpG to albumin cysteine 34, distant from its main FcRn-binding interface, resulted in a retained pH-dependent human FcRn binding, and receptor-driven endosomal trafficking in a cellular recycling assay. Induction of tumor necrosis factor (TNF) secretion in THP-1 cells and interferon alpha (IFN-α) in human hematopoietic stem and progenitor cell (HSPC)-derived plasmacytoid dendritic cells (pDCs), in contrast, to a myeloid differentiation primary response 88 (MyD88) and TLR9 knockout cells, respectively, support TLR9-engagement. The rHA-CpG construct induced greater TNF-α than free CpG ODN in mouse RAW 264.7 cells, and in human peripheral blood mononuclear cells (PBMCs) and expansion of classical (CD14+CD16-) monocytes. Furthermore, greater accumulation of Cy5.5-labelled CpG in the inguinal (>3-fold) and axillary (>18-fold) lymph nodes was observed when conjugated to rHA compared to an unconjugated rHA/CpG mix following subcutaneous injection in mice. Moreover, increased LN accumulation of an rHA variant engineered with high FcRn-binding affinity supports an FcRn-driven mechanism. Demonstration of FcRn-mediated albumin targeting at intra- and extracellular sites provides the mechanistic basis for the potent immune induction observed using the novel rHA-CpG conjugate design class introduced in this work.
Keywords: CpG ODN; FcRn; TLR9; albumin; immune adjuvant; innate immunity; lymph nodes.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kenneth A Howard and Diego Pilati are co-inventors of a patent application on technology described in the paper.
Figures





References
-
- Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdörfer B., Giese T., et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

