Externally validated digital decision support tool for time-to-osteoradionecrosis risk-stratification using right-censored multi-institutional observational cohorts
- PMID: 40222595
- PMCID: PMC12085279
- DOI: 10.1016/j.radonc.2025.110890
Externally validated digital decision support tool for time-to-osteoradionecrosis risk-stratification using right-censored multi-institutional observational cohorts
Abstract
Background: Existing studies on osteoradionecrosis of the jaw (ORNJ) have primarily used cross-sectional data, assessing risk factors at a single time point. Determining the time-to-event profile of ORNJ has important implications to monitor oral health in head and neck cancer (HNC) long-term survivors.
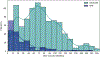
Methods: Data were retrospectively obtained for a clinical observational cohort of 1129 patients (198 ORNJ cases) with HNC treated with radiotherapy (RT) at The University of Texas MD Anderson Cancer Center. A Weibull Accelerated Failure Time model was trained on previously identified dosimetric, clinical and demographic predictors. External validation was performed using an independent cohort of 265 patients (92 ORNJ cases) treated at Guy's and St. Thomas' Hospitals. To facilitate clinical implementation of the model, an online graphical user interface (GUI) was developed, including formal stakeholder usability testing.
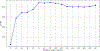
Results: Our model identified that gender (males), pre-RT dental extractions and D25% were associated with a 38 %, 27 % and 12 % faster onset of ORNJ, respectively, with adjusted time ratios of 0.62 (p = 0.11), 0.73 (p = 0.13) and 0.88 (p < 0.005). The model demonstrated strong internal calibration (integrated Brier score of 0.133, D-calibration p-value 0.998) and optimal discrimination at 72 months (Harrell's C-index of 0.72).
Conclusion: This study is the first to demonstrate a direct relationship between radiation dose and the time to ORNJ onset, providing a novel characterization of the impact of delivered dose and patient-related factors not only on the probability of a late effect (ORNJ), but the conditional risk during survivorship.
Keywords: Decision support tool; Head and neck cancers; Normal tissue complication prediction; Osteoradionecrosis of the jaw; Radiation-induced toxicity; Radiotherapy; Time-to-event prediction models.
Copyright © 2025. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CDF has received unrelated grant support from Elekta AB and holds unrelated patents licensed to Kallisio, Inc. (US PTO 11730561) through the University of Texas, from which they receive patent royalties. CDF has also received unrelated travel and honoraria from Elekta AB, Philips Medical Systems, Siemens Healthineers/Varian, and Corewell Health. Additionally, CDF has served in an unpaid advisory capacity for Siemens Healthineers/Varian and has served on the guidelines/scientific committee for Osteoradionecrosis for the American Society of Clinical Oncology. VCS is a consultant and equity holder in Femtovox Inc and a consultant for PDS Biotechnology. KAW serves as an Editorial Board Member for Physics and Imaging in Radiation Oncology. The authors declare that no other competing interests exist.
Figures
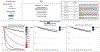






References
-
- Habib S; Sassoon I; Thompson I; Patel V Risk Factors Associated with Osteoradionecrosis. Oral Surgery 2021, 14 (3), 227–235. 10.1111/ors.12597. - DOI
-
- MD Anderson Head and Neck Cancer Symptom Working Group. Dose-Volume Correlates of Mandibular Osteoradionecrosis in Oropharynx Cancer Patients Receiving Intensity-Modulated Radiotherapy: Results from a Case-Matched Comparison. Radiother Oncol 2017, 124 (2), 232–239. 10.1016/j.radonc.2017.06.026. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- T32 CA261856/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 DE025248/DE/NIDCR NIH HHS/United States
- U01 DE032168/DE/NIDCR NIH HHS/United States
- UG3 TR004501/TR/NCATS NIH HHS/United States
- K01 DE030524/DE/NIDCR NIH HHS/United States
- P01 CA285249/CA/NCI NIH HHS/United States
- R01 CA258827/CA/NCI NIH HHS/United States
- R01 DE028290/DE/NIDCR NIH HHS/United States
- P50 CA221707/CA/NCI NIH HHS/United States
- R01 CA257814/CA/NCI NIH HHS/United States
- R21 DE031082/DE/NIDCR NIH HHS/United States
- K12 CA088084/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

