Rap2B drives tumorigenesis and progression of colorectal cancer through intestinal cytoskeleton remodeling
- PMID: 40223002
- PMCID: PMC11994759
- DOI: 10.1038/s41419-025-07627-8
Rap2B drives tumorigenesis and progression of colorectal cancer through intestinal cytoskeleton remodeling
Abstract
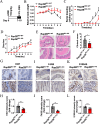
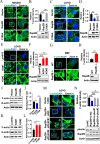
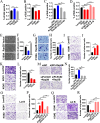
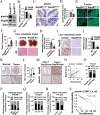
Ras family protein plays a key role in transducing signals involved in cytoskeletal remodeling and cell adhesion, which are particularly important in the development of colorectal cancer (CRC). While Rap2B, a member of the Ras superfamily, has been linked to cancer malignancy in vitro, its exact role in tumorigenesis remains unclear. In this study, we demonstrated that intestine-specific knockout of Rap2B suppresses the initiation and progression of CRC. Mechanistically, Rap2B interacts with plectin and enhances its expression, which in turn inhibits plectin-mediated F-actin assembly. Deletion of Rap2B resulted in a remodeling of the intestinal cytoskeleton, leading to reduced tumorigenesis and diminished metastatic potential. Clinically, there is a positive correlation between the expression levels of Rap2B and plectin in human CRC tissues, and higher levels of Rap2B and plectin predicting poorer clinical outcome in CRC patients. These findings underscore a critical role of Rap2B in CRC progression and highlight its potential as a therapeutic target.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

