Anti-T lymphocyte globulin plus posttransplant cyclophosphamide 25 mg/kg versus posttransplant cyclophosphamide 50 mg/kg in patients with acute leukemias
- PMID: 40223010
- PMCID: PMC12151847
- DOI: 10.1038/s41409-025-02564-8
Anti-T lymphocyte globulin plus posttransplant cyclophosphamide 25 mg/kg versus posttransplant cyclophosphamide 50 mg/kg in patients with acute leukemias
Abstract
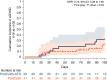
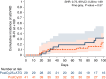
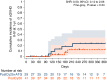
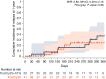
In this study, we aimed to compare the engraftment days, graft-versus-host disease (GVHD) development, relapse and overall survival rates in patients using myeloablative/reduced intensity conditioning regimens with posttransplant cyclophosphamide (PTCy) 25 mg/kg x2 with Anti-T lymphocyte Globulin (ATLG) (n = 29) and PTCy 50 mg/kg x2 doses (n = 41) in patients with acute leukemias. Matched related, matched unrelated, 1 mismatched unrelated, and haploidentical donors were selected for the patients. Platelet (median 11 vs 17 days) and neutrophil (median 14 vs 15 days) engraftment times were shorter in ATLG+ PTCy25 treated patients (both p < 0.05); veno-occlusive disease rates, graft failure and poor graft functions were similar between the two approaches (all p > 0.05); cumulative incidences of grade II-IV aGVHD at +100 days, grade III-IV aGVHD at +100 days, and grade II-IV cGVHD at 1-year were comparable between ATLG+PTCy25 and PTCy50 groups (all p > 0.05). Cumulative incidences of relapse and non-relapse mortality at 1-year were similar in two cohorts (both p > 0.05). PTCy50 was associated with a statistically significant benefit in terms of GVHD-free/relapse-free survival (GRFS) at 1-year (p = 0.03). Median GRFS was 115 (95% CI: 42-214) days and 248 (95% CI: 151-not reached) days, respectively [HR was 0.51 (0.28-0.95), p = 0.03; GRFS at 1-year was 20.7% vs 44.3%, respectively]. However, the groups were comparable in terms of PFS and OS. Median PFS was 332 days (95% CI: 182 days-not reached) for ATLG+PTCy25 group. It was not reached (95% CI: 210 days-not reached) for the patients who received PTCy50. Median OS was not reached in either ATLG+PTCy25 (95% CI: 191 days-not reached) or PTCy50 groups (Log rank = 0.42). Our study showed that lowering PTCy dose with ATLG seems to accelerate platelet and neutrophil engraftment rates; confers similar survival and relapse rates, similar acute and chronic GVHD frequency despite increased GRFS at 1-year.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
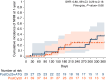
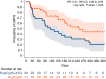
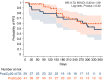
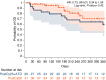
Similar articles
-
Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients.J Hematol Oncol. 2019 Sep 3;12(1):88. doi: 10.1186/s13045-019-0781-y. J Hematol Oncol. 2019. PMID: 31481121 Free PMC article.
-
Post-transplant cyclophosphamide with post-engraftment anti-thymocyte globulin reduce moderate to severe chronic graft-versus-host disease in peripheral stem cell transplantation from HLA-matched unrelated and haploidentical donors.Bone Marrow Transplant. 2025 Jan;60(1):58-63. doi: 10.1038/s41409-024-02436-7. Epub 2024 Oct 22. Bone Marrow Transplant. 2025. PMID: 39433913
-
Anti-T-lymphocyte globulin (ATLG) compared to post-transplant cyclophosphamide as GvHD prophylaxis in ALL patients undergoing allogeneic stem cell transplantation.Bone Marrow Transplant. 2024 Sep;59(9):1265-1274. doi: 10.1038/s41409-024-02328-w. Epub 2024 Jun 14. Bone Marrow Transplant. 2024. PMID: 38877098 Free PMC article.
-
Uniform Graft-versus-Host Disease Prophylaxis using Post-Transplantation Cyclophosphamide, Methotrexate, and Cyclosporine following Peripheral Blood Hematopoietic Stem Cell Transplantation from Matched and Haploidentical Donors for Transfusion-Dependent Thalassemia: A Retrospective Report from the Bone Marrow Failure Working Group of Hunan Province, China.Transplant Cell Ther. 2024 Dec;30(12):1213.e1-1213.e12. doi: 10.1016/j.jtct.2024.08.022. Epub 2024 Sep 3. Transplant Cell Ther. 2024. PMID: 39236789
-
Outcomes after Haploidentical Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide: A Systematic Review and Meta-Analysis Comparing Myeloablative with Reduced-Intensity Conditioning Regimens and Bone Marrow with Peripheral Blood Stem Cell Grafts.Transplant Cell Ther. 2021 Sep;27(9):782.e1-782.e7. doi: 10.1016/j.jtct.2021.06.011. Epub 2021 Jun 16. Transplant Cell Ther. 2021. PMID: 34146733
References
-
- Abedin S, Hamadani M. Contemporary updates in the prevention and treatment of graft-versus-host disease. Curr Hematol Malig Rep. 2024;19:246–55. 10.1007/s11899-024-00741-y - PubMed
-
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–63. 10.1016/j.bbmt.2012.04.005 - PMC - PubMed
-
- Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, et al. Haemato-oncology task force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45. 10.1111/j.1365-2141.2012.09129.x - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

