Leukoreduction filter derived NK cells offer a promising source for off the shelf CAR NK cell immunotherapy
- PMID: 40223011
- PMCID: PMC11994799
- DOI: 10.1038/s41598-025-97584-1
Leukoreduction filter derived NK cells offer a promising source for off the shelf CAR NK cell immunotherapy
Abstract
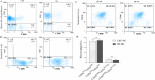
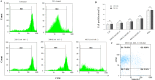
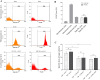
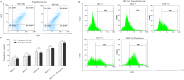
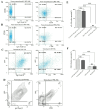
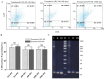
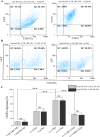
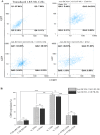
Immunotherapy employing natural killer (NK) cells has emerged as a transformative approach to treating hematological malignancies. The reprogramming of NK cells by incorporating a chimeric antigen receptor (CAR) equipped with potent signaling domains has demonstrated efficacy in enhancing NK cell responses and improving specificity in recognizing cancerous cells. Despite these advancements, the primary challenge in implementing allogeneic NK cell therapy requiring a viable donor source for clinically relevant doses remains unresolved. This study tested NK cells obtained from leukoreduction filters (LRF) post-blood donation to address the need for an efficient and scalable supply of NK cells for generating anti-BCMA CAR NK cells. LRF-NK cells were isolated under sterile conditions and compared with peripheral blood (PB)-derived NK cells in terms of immunophenotype, proliferation capacity, and functional characteristics. Notably, no significant differences in inherent characteristics were observed between LRF-NK and PB-NK cells. Subsequently, both NK cell populations were employed to generate anti-BCMA CAR-NK cells. The data revealed a high specific cytotoxicity of Anti-BCMA CAR LRF-NK cells during co-culture with U266-B1 cells (70.3 ± 4.78%), surpassing that observed with CCRF-CEM cells (31.3 ± 2.35%) and similar to Anti-BCMA CAR PB-NK cells. Furthermore, the expression of IFN-γ and Granzyme B, following the co-culture of Anti-BCMA CAR LRF-NK cells with target cells, mirrored that observed in Anti-BCMA CAR PB-NK cells. This study provides the rationale and feasibility of utilizing LRF-NK cells as a safe, high-yield, accessible, and optimal cost-effective source for cancer immunotherapy.
Keywords: Chimeric antigen receptor (CAR); Healthy donor; Leukoreduction filters (LRF); Natural killer (NK) cell.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells.J Transl Med. 2022 Mar 14;20(1):124. doi: 10.1186/s12967-022-03326-6. J Transl Med. 2022. PMID: 35287669 Free PMC article.
-
CXCR4 has a dual role in improving the efficacy of BCMA-redirected CAR-NK cells in multiple myeloma.Front Immunol. 2024 Jun 24;15:1383136. doi: 10.3389/fimmu.2024.1383136. eCollection 2024. Front Immunol. 2024. PMID: 38979422 Free PMC article.
-
Preclinical Assessment of Suitable Natural Killer Cell Sources for Chimeric Antigen Receptor Natural Killer-Based "Off-the-Shelf" Acute Myeloid Leukemia Immunotherapies.Hum Gene Ther. 2019 Apr;30(4):381-401. doi: 10.1089/hum.2018.247. Epub 2019 Mar 18. Hum Gene Ther. 2019. PMID: 30734584
-
[Allogeneic CAR-NK cells: A promising alternative to autologous CAR-T cells - State of the art, sources of NK cells, limits and perspectives].Bull Cancer. 2021 Oct;108(10S):S81-S91. doi: 10.1016/j.bulcan.2021.06.007. Bull Cancer. 2021. PMID: 34920811 Review. French.
-
CAR-NK cell in cancer immunotherapy; A promising frontier.Cancer Sci. 2021 Sep;112(9):3427-3436. doi: 10.1111/cas.14993. Epub 2021 Jul 7. Cancer Sci. 2021. PMID: 34050690 Free PMC article. Review.
References
-
- Feins, S., Kong, W., Williams, E. F., Milone, M. C. & Fraietta, J. A. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol.94(S1), S3–S9 (2019). - PubMed
-
- Miliotou, A. N. & Papadopoulou, L. C. CAR T-cell therapy: a new era in cancer immunotherapy. Curr. Pharm. Biotechnol.19(1), 5–18 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

