Production of alkaline lipase by Aspergillus terreus AUMC 15762 for laundry application
- PMID: 40223016
- PMCID: PMC11994561
- DOI: 10.1186/s13568-025-01865-x
Production of alkaline lipase by Aspergillus terreus AUMC 15762 for laundry application
Abstract
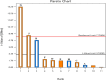
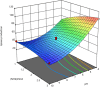
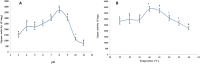
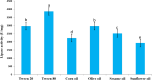
Lipases are extraordinarily critical co-factor-independent enzymes with profound economic consequences. They are utilized extensively in production of fine chemicals, food, textile, pulp and paper, laundry, and biodiesel sectors. In the current study, the lipolytic activity of 141 fungal isolates-representing 21 genera and 38 species-that were isolated from samples of desert soil gathered from the Governorates of Sohag, Qena, and Aswan were examined. Of the 74 isolates showed positive lipase activity, 40 were high lipase producers. In terms of lipase production, Aspergillus terreus AUMC 15762 was the most effective strain. To enhance the synthesis of lipase from Aspergillus terreus AUMC 15762, Plackett-Burman design (PBD) was employed. For the maximal amount of lipase synthesis (103.3 U/mL), ammonium sulphate was required after three days at 25 °C, pH 4.0, and 3.0 g/L. Through the use of Trilite MA 12 anion exchanger and Sephadex G-100 column chromatography, lipase was purified 17.79 times and achieved 64.714 kDa molecular weight on SDS-PAGE. The highest possible specific activity of 3867.85 ± 214.28 U/mg was attained at pH 8.0 and 40 °C. The addition of KCl and ZnSO4 raised the lipase specific activity by 115.42%. Km of 19.0 mg/mL and Vmax of 1000 μmol/min were determined for the pure lipase. The effects of 20 U/mL pure lipase on corn and olive oily spots were examined in this work at pH 8.0 and 40 °C. The pure lipase completely removed oil contamination from fiber surfaces, as evidenced by the oily spots' separation from the white cotton textiles after 60 min. This work offers a lipase produced from Aspergillus terreus species that showed promise for industrial laundry applications.
Keywords: Aspergillus; Laundry; Lipase; Optimization; Plackett–Burman; Purification; Submerged.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Thermo-alkali-stable lipase from a novel Aspergillus niger: statistical optimization, enzyme purification, immobilization and its application in biodiesel production.Prep Biochem Biotechnol. 2021;51(3):225-240. doi: 10.1080/10826068.2020.1805759. Epub 2020 Aug 18. Prep Biochem Biotechnol. 2021. PMID: 32808876
-
Optimization of lipase production using fungal isolates from oily residues.BMC Biotechnol. 2021 Nov 10;21(1):65. doi: 10.1186/s12896-021-00724-4. BMC Biotechnol. 2021. PMID: 34758800 Free PMC article.
-
Optimization of process parameters influencing the submerged fermentation of extracellular lipases from Pseudomonas aeruginosa, candida albicans and Aspergillus flavus.Pak J Biol Sci. 2011 Nov 15;14(22):1011-8. doi: 10.3923/pjbs.2011.1011.1018. Pak J Biol Sci. 2011. PMID: 22514878
-
Purification and characterization of a hydrolysis-resistant lipase from Aspergillus terreus.Biotechnol Appl Biochem. 2014 Mar-Apr;61(2):165-74. doi: 10.1002/bab.1142. Epub 2013 Nov 21. Biotechnol Appl Biochem. 2014. PMID: 23855368
-
Overview of fungal lipase: a review.Appl Biochem Biotechnol. 2012 Jan;166(2):486-520. doi: 10.1007/s12010-011-9444-3. Epub 2011 Nov 10. Appl Biochem Biotechnol. 2012. PMID: 22072143 Review.
References
-
- Al-Bedak OA, Moubasher AH (2020) Aspergillus gaarensis, a new addition to section Circumdati from soil of Lake El-Gaar in Wadi-El-Natron Egypt. Stud Fungi 5(1):59–65
-
- Bae J-H, Kwon M-H, Kim I-H, Hou CT, Kim H-R (2014) Purification and characterization of a cold-active lipase from Pichia lynferdii Y-7723: pH-dependant activity deviation. Biotechnol Bioprocess Eng 19:851–857
LinkOut - more resources
Full Text Sources

