Biomarkers of ocular manifestation in newly diagnosed giant cell arteritis
- PMID: 40223083
- PMCID: PMC11995548
- DOI: 10.1186/s12886-025-03997-x
Biomarkers of ocular manifestation in newly diagnosed giant cell arteritis
Abstract
Background: Giant cell arteritis (GCA) is a vasculitis of large and medium-sized vessels that causes severe ophthalmic complications. Timely diagnosis and disease monitoring may prevent permanent vision loss but biomarkers for early ocular involvement are scarce. This study evaluates early optical coherence tomography (OCT) and OCT angiography (OCTA) biomarkers of ocular involvement in newly diagnosed GCA.
Methods: Newly diagnosed GCA patients and similarly aged controls were enrolled. Participants underwent ocular examination, including OCT and OCTA imaging of the macula and optic disc. OCT metrics included macular ganglion cell layer (GCL) and peripapillary retinal nerve fiber layer (pRNFL) thickness. OCTA parameters included vessel density (VD), vessel skeleton density, and vessel diameter index (VDI). Associations between imaging biomarkers and GCA symptoms (ordinal GCA symptom score) were analyzed using age-adjusted regression models.
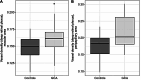
Results: We recruited 23 newly diagnosed GCA patients and 27 controls. VD and VDI in the deep retinal capillaries were significantly higher in GCA compared to controls (p ≤ 0.027). In patients reporting ocular symptoms, GCL thickness, volume and pRNFL thickness were increased in 11%, 22% and 17% of Early Treatment Diabetic Retinopathy Study subfields compared to controls (p ≤ 0.04). Additionally, GCL and pRNFL thicknesses were associated with GCA symptoms (p ≤ 0.041).
Conclusions: OCT and OCTA imaging revealed structural and perfusion alterations in newly diagnosed GCA patients. Retinal microcirculation was altered, even regardless of the presence of ophthalmic symptoms. Structural changes correlated with systemic manifestations of GCA, suggesting a link between extracranial and intracranial involvement. Our findings underscore the potential diagnostic value of OCT and OCTA biomarkers for ocular involvement in GCA.
Keywords: Giant cell arteritis; Imaging; Ocular biomarker; Optical coherence tomography; Optical coherence tomography angiography.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and received approval from the ethics committee of the University Hospital Bonn, Germany (reference number: 097/18). Written informed consent was obtained from every patient prior to inclusion in the study. Consent for publication: Not applicable. Competing interests: JHT: Bayer (Consulting, Funding, Honoraria); Novartis (Recipient, Honoraria), Roche (Funding), Okko (Consulting) SMP, LCB, VSS none JJ: None KR: None FGH: Acucela (Consulting, Funding), Allergan (Funding), Apellis (Consulting, Funding), Bayer (Consulting, Funding), Boehringer-Ingelheim (Consulting), Bioeq/ Formycon (F,C), CenterVue (Funding), Ellex (Funding), Roche/Genentech (Consulting, Funding), Geuder (Consulting, Funding), Graybug (Consulting), Gyroscope (Consulting), Heidelberg Engineering (Consulting, Funding), IvericBio (Consulting, Funding), Kanghong (Consulting, Funding), LinBioscience (Consulting), NightStarX (Funding), Novartis (Consulting, Funding), Optos (Funding), Oxurion (Consulting), Pixium Vision (Consulting, Funding), Oxurion (Consulting), Stealth BioTherapeutics (Consulting), Zeiss (Funding, Consulting) TA: Novartis (Speaker honorarium), Astellas (Speaker honorarium, Advisory board), Apellis (Speaker honorarium), Heidelberg Engineering (Speaker honorarium), Bayer (Speaker honorarium), Roche (Speaker honorarium, Travel reimbursement), Nidek (Research funding, speaker honorarium) MWMW: Baxter Healthcare Corporation: research grant, Bayer AG: honoraria, glaucare GmbH: consultant, owner , Novartis Pharma GmbH: honoraria, grant, DigiSight Technologies: travel grant, D-EYE Srl: imaging devices, Heine Optotechnik GmbH: research funding, imaging devices, travel reimbursements, consultant; Eyenuk, Inc: free trial analysis; ASKIN & CO GmbH: travel reimbursement, honoraria; Berlin-Chemie AG: grant, travel reimbursements; Imaging devices were provided by Heidelberg Engineering, Optos, Carl Zeiss Meditec, and CenterVue RPF: Bayer (Consultant), Ellex (Consultant), Novartis (Consultant), Novartis (Funding), Ophtea (Consultant), Alimera (Consultant), Santhera (Consultant), Roche/Genentech (Consultant), ICare (formerly CentreVue) (Funding), Zeiss (Funding)
Figures
References
-
- Patil P, Williams M, Maw WW, Achilleos K, Elsideeg S, Dejaco C, et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol. 2015;33:S–103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical