Florfenicol-induced dysbiosis impairs intestinal homeostasis and host immune system in laying hens
- PMID: 40223090
- PMCID: PMC11995664
- DOI: 10.1186/s40104-025-01186-w
Florfenicol-induced dysbiosis impairs intestinal homeostasis and host immune system in laying hens
Abstract
Background: Despite growing concerns about the adverse effects of antibiotics in farm animals, there has been little investigation of the effects of florfenicol in laying hens. This study examined the effect of florfenicol on the intestinal homeostasis, immune system, and pathogen susceptibility of laying hens.
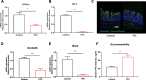
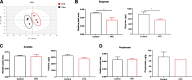
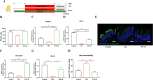
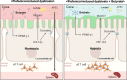
Results: The oral administration of florfenicol at field-relevant levels for 5 d resulted in a decrease in the gut microbiota genera Lactobacillus, Bacillus, and Bacteroides, indicating the development of intestinal dysbiosis. The dysbiosis led to decreased mRNA levels of key regulators peroxisome proliferator-activated receptor gamma (PPAR-γ) and hypoxia-inducible factor-1α (HIF-1α), compromising intestinal hypoxia. Intestinal homeostasis was also disrupted, with decreased expression of Occludin and Mucin 2 (Muc2) genes combined with increased gut epithelial permeability. The breakdown in intestinal homeostasis and immune function provided a favorable environment for opportunistic bacteria like avian pathogenic Escherichia coli (APEC), culminating in systemic infection. Immunologically, florfenicol treatment resulted in increased proportion and absolute number of MRC1L-B+ monocytes/macrophages in the spleen, indicating an exacerbated infection. Furthermore, both the proportion and absolute number of γδ T cells in the lamina propria of the cecum decreased. Treatment with florfenicol reduced butyrate levels in the cecum. However, the administration of butyrate before and during florfenicol treatment restored factors associated with intestinal homeostasis, including PPAR-γ, Occludin, and Muc2, while partially restoring HIF-1α, normalized intestinal hypoxia and gut permeability, and reversed immune cell changes, suppressing APEC systemic infection.
Conclusion: The uncontrolled and widespread use of florfenicol can negatively affect intestinal health in chickens. Specifically, florfenicol was found to impair intestinal homeostasis and immune function in laying hens, including by reducing butyrate levels, thereby increasing their susceptibility to systemic APEC infection. The development of strategies for mitigating the adverse effects of florfenicol on gut health and pathogen susceptibility in laying hens is therefore essential.
Keywords: Antibiotics-induced dysbiosis; Avian immunology; Avian pathogenic Escherichia coli; Gut homeostasis; Laying hen; Short chain fatty acids.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experiment was approved by the Institutional Animal Care and Use Committee of Seoul National University (IACUC No.: SNU-230818-1). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




Similar articles
-
Effects of dustbathing environment on gut microbiota and expression of intestinal barrier and immune-related genes of adult laying hens housed individually in modified traditional cage.Poult Sci. 2023 Dec;102(12):103097. doi: 10.1016/j.psj.2023.103097. Epub 2023 Sep 9. Poult Sci. 2023. PMID: 37769487 Free PMC article.
-
Improving fatty liver hemorrhagic syndrome in laying hens through gut microbiota and oxylipin metabolism by Bacteroides fragilis: A potential involvement of arachidonic acid.Anim Nutr. 2024 Nov 1;20:182-199. doi: 10.1016/j.aninu.2024.08.008. eCollection 2025 Mar. Anim Nutr. 2024. PMID: 39967692 Free PMC article.
-
Supplementation of curcumin promotes the intestinal structure, immune barrier function and cecal microbiota composition of laying hens in early laying period.Poult Sci. 2024 Dec;103(12):104355. doi: 10.1016/j.psj.2024.104355. Epub 2024 Sep 24. Poult Sci. 2024. PMID: 39423789 Free PMC article.
-
Predisposition factors and control strategies of avian pathogenic Escherichia coli in laying hens.Front Vet Sci. 2024 Nov 4;11:1474549. doi: 10.3389/fvets.2024.1474549. eCollection 2024. Front Vet Sci. 2024. PMID: 39559543 Free PMC article. Review.
-
The Impact of Probiotic Bacillus subtilis on Injurious Behavior in Laying Hens.Animals (Basel). 2022 Mar 30;12(7):870. doi: 10.3390/ani12070870. Animals (Basel). 2022. PMID: 35405859 Free PMC article. Review.
References
Grants and funding
- RS-2023-00218476/National Research Foundation (KR)
- RS-2024-00454619/National Research Foundation (KR)
- RS-2022-RD010165/the Cooperative Research Program for Agriculture Science and Technology Development (KR)
- RS-2022- KH128577/the Ministry of Health & Welfare (KR)
- Department of Agricultural Biotechnology/the BK21 FOUR Program (KR)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

