Novel strategies for vancomycin-resistant Enterococcus faecalis biofilm control: bacteriophage (vB_EfaS_ZC1), propolis, and their combined effects in an ex vivo endodontic model
- PMID: 40223105
- PMCID: PMC11995525
- DOI: 10.1186/s12941-025-00790-y
Novel strategies for vancomycin-resistant Enterococcus faecalis biofilm control: bacteriophage (vB_EfaS_ZC1), propolis, and their combined effects in an ex vivo endodontic model
Abstract
Background: Endodontic treatment failures are predominantly attributed to Enterococcus faecalis (E. faecalis) infection, a Gram-positive coccus. E. faecalis forms biofilms, resist multiple antibiotics, and can withstand endodontic disinfection protocols. Vancomycin-resistant strains, in particular, are challenging to treat and are associated with serious medical complications.
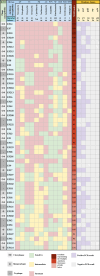
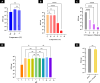
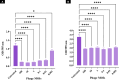
Methods: A novel phage, vB_EfaS_ZC1, was isolated and characterized. Its lytic activity against E. faecalis was assessed in vitro through time-killing and biofilm assays. The phage's stability under various conditions was determined. Genomic analysis was conducted to characterize the phage and its virulence. The phage, propolis, and their combination were evaluated as an intracanal irrigation solution against a 4-week E. faecalis mature biofilm, using an ex vivo infected human dentin model. The antibiofilm activity was analyzed using a colony-forming unit assay, field emission scanning electron microscopy, and confocal laser scanning microscopy.
Results: The isolated phage, vB_EfaS_ZC1, a siphovirus with prolate capsid, exhibited strong lytic activity against Vancomycin-resistant strains. In vitro assays indicated its effectiveness in inhibiting planktonic growth and disrupting mature biofilms. The phage remained stable under wide range of temperatures (- 80 to 60 °C), tolerated pH levels from 4 to 11; however the phage viability significantly reduced after UV exposure. Genomic analysis strongly suggests the phage's virulence and suitability for therapeutic applications; neither lysogeny markers nor antibiotic resistance markers were identified. Phylogenetic analysis clustered vB_EfaS_ZC1 within the genus Saphexavirus. The phage, both alone and in combination with propolis, demonstrated potent antibiofilm effects compared to conventional root canal irrigation.
Conclusion: Phage vB_EfaS_ZC1 demonstrates a promising therapy, either individually or in combination with propolis, for addressing challenging endodontic infections caused by E. faecalis.
Keywords: Saphexavirus; Antibiofilm; Endodontic treatment; Irrigation; Phage therapy; Propolis; Vancomycin-resistant Enterococcus faecalis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Preferred Reporting Items for Laboratory Studies in Endodontology (PRILE) 2021 standards were followed in writing the paper for this laboratory study. This study was approved by the Research Ethics Committee (REC), Number 703/2023. Faculty of Dentistry, Suez Canal University, Ismailia, Egypt. Established according to “WHO-2011” standards (Date of approval: 3 October 2023). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures





Similar articles
-
Enterococcus Phage vB_EfaS_HEf13 as an Anti-Biofilm Agent Against Enterococcus faecalis.J Microbiol. 2024 Aug;62(8):683-693. doi: 10.1007/s12275-024-00150-z. Epub 2024 Jun 27. J Microbiol. 2024. PMID: 38935316
-
Therapeutic potential of a newly isolated bacteriophage against multi-drug resistant Enterococcus faecalis infections: in vitro and in vivo characterization.BMC Microbiol. 2025 Feb 20;25(1):80. doi: 10.1186/s12866-025-03785-z. BMC Microbiol. 2025. PMID: 39979834 Free PMC article.
-
Characterization and Anti-Biofilm Activity of Lytic Enterococcus Phage vB_Efs8_KEN04 against Clinical Isolates of Multidrug-Resistant Enterococcus faecalis in Kenya.Viruses. 2024 Aug 9;16(8):1275. doi: 10.3390/v16081275. Viruses. 2024. PMID: 39205249 Free PMC article.
-
Strategies and mechanisms targeting Enterococcus faecalis biofilms associated with endodontic infections: a comprehensive review.Front Cell Infect Microbiol. 2024 Jul 18;14:1433313. doi: 10.3389/fcimb.2024.1433313. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39091674 Free PMC article. Review.
-
Bacteriophage therapy for critical antibiotic-resistant Gram-positive bacteria: A systematic review of clinical researches.Microbiol Res. 2025 Sep;298:128231. doi: 10.1016/j.micres.2025.128231. Epub 2025 May 22. Microbiol Res. 2025. PMID: 40424685 Review.
References
-
- Haapasalo M, et al. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Top. 2005;10(1):77–102.
-
- van Harten RM, et al. Multidrug-resistant Enterococcal Infections: New compounds, novel antimicrobial therapies? Trends Microbiol. 2017;25(6):467–79. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

