BIRC2 blockade facilitates immunotherapy of hepatocellular carcinoma
- PMID: 40223121
- PMCID: PMC11995630
- DOI: 10.1186/s12943-025-02319-5
BIRC2 blockade facilitates immunotherapy of hepatocellular carcinoma
Abstract
Background: The effectiveness of immunotherapy in hepatocellular carcinoma (HCC) is limited, however, the molecular mechanism remains unclear. In this study, we identified baculoviral IAP repeat-containing protein 2 (BIRC2) as a key regulator involved in immune evasion of HCC.
Methods: Genome-wide CRISPR/Cas9 screening was conducted to identify tumor-intrinsic genes pivotal for immune escape. In vitro and in vivo models demonstrated the role of BIRC2 in protecting HCC cells from immune killing. Then the function and relevant signaling pathways of BIRC2 were explored. The therapeutic efficacy of BIRC2 inhibitor was examined in different in situ and xenograft HCC models.
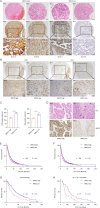
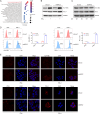
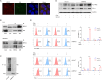
Results: Elevated expression of BIRC2 correlated with adverse prognosis and resistance to immunotherapy in HCC patients. Mechanistically, BIRC2 interacted with and promoted the ubiquitination-dependent degradation of NFκB-inducing kinase (NIK), leading to the inactivation of the non-canonical NFκB signaling pathway. This resulted in the decrease of major histocompatibility complex class I (MHC-I) expression, thereby protecting HCC cells from T cell-mediated cytotoxicity. Silencing BIRC2 using shRNA or inhibiting it with small molecules increased the sensitivity of HCC cells to immune killing. Meanwhile, BIRC2 blockade improved the function of T cells both in vitro and in vivo. Targeting BIRC2 significantly inhibited tumor growth, and enhanced the efficacy of anti-programmed death protein 1 (PD-1) therapy.
Conclusions: Our findings suggested that BIRC2 blockade facilitated immunotherapy of HCC by simultaneously sensitizing tumor cells to immune attack and boosting the anti-tumor immune response of T cells.
Keywords: BIRC2; Hepatocellular carcinoma; Immunotherapy; MHC-I; NIK.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval was granted by the Sun Yat-sen University Cancer Center Institute Research Ethics Committee, and all procedures adhered to the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS) (GZR2022-054). Written informed consent was obtained from each patient or their legal guardians. Ethical approval for all animal procedures was granted by the Institutional Animal Care and Use Committee of Sun Yat-sen University (L102012022005T). Competing interests: The authors declare no competing interests.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. - PubMed
-
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

