Observation of the Assembly of the Nascent Mineral Core at the Nucleation Site of Human Mitochondrial Ferritin
- PMID: 40223208
- PMCID: PMC12022971
- DOI: 10.1021/jacs.5c01337
Observation of the Assembly of the Nascent Mineral Core at the Nucleation Site of Human Mitochondrial Ferritin
Abstract
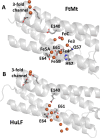
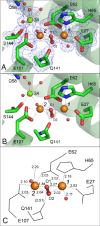
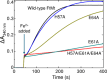
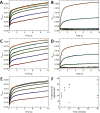
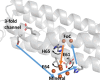
Ferritins play a crucial role in iron homeostasis and detoxification in organisms from all kingdoms of life. They are composed of 24 α-helical subunits arranged around an interior cavity where an iron-containing mineral core can be reversibly stored. Despite decades of study, leading to significant progress in defining the routes of Fe2+ uptake and the mechanism of its subsequent oxidation to Fe3+ at diiron catalytic sites termed ferroxidase centers, the process of core synthesis from the product of ferroxidase center activity remains poorly understood. In large part, this is due to the lack of high-resolution structural data on ferritin cores anchored to their nucleation sites on the inner surface of the protein. Mitochondrial ferritins are atypical of those found in higher eukaryotes in that they are homopolymers in which all subunits contain both a ferroxidase center and a presumed but undefined core nucleation site. Here, in conjunction with a novel method for producing iron-enriched ferritin crystals, we exploit these unusual features to structurally characterize both the nucleation site of mitochondrial ferritin and a pentanuclear, ferrihydrite-like iron-oxo cluster formed there. Kinetic data for wild-type and variant proteins confirmed the functional importance of this site, indicating a critical role for E61 in the transfer of Fe3+ from the ferroxidase center to the nascent mineral core.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
The iron redox and hydrolysis chemistry of the ferritins.Biochim Biophys Acta. 2010 Aug;1800(8):719-31. doi: 10.1016/j.bbagen.2010.03.021. Epub 2010 Apr 9. Biochim Biophys Acta. 2010. PMID: 20382203 Review.
-
The putative "nucleation site" in human H-chain ferritin is not required for mineralization of the iron core.Biochemistry. 2004 Apr 13;43(14):4332-7. doi: 10.1021/bi0498813. Biochemistry. 2004. PMID: 15065877
-
Catalysis of iron core formation in Pyrococcus furiosus ferritin.J Biol Inorg Chem. 2009 Nov;14(8):1265-74. doi: 10.1007/s00775-009-0571-z. Epub 2009 Jul 22. J Biol Inorg Chem. 2009. PMID: 19623480 Free PMC article.
-
Key carboxylate residues for iron transit through the prokaryotic ferritin SynFtn.Microbiology (Reading). 2021 Nov;167(11):001105. doi: 10.1099/mic.0.001105. Microbiology (Reading). 2021. PMID: 34825885 Free PMC article.
-
Mineralization in ferritin: an efficient means of iron storage.J Struct Biol. 1999 Jun 30;126(3):182-94. doi: 10.1006/jsbi.1999.4118. J Struct Biol. 1999. PMID: 10441528 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

