Photoelectrochemical Iron(III) Catalysis for Late-Stage C─H Fluoroalkylations
- PMID: 40223224
- PMCID: PMC12171357
- DOI: 10.1002/anie.202504143
Photoelectrochemical Iron(III) Catalysis for Late-Stage C─H Fluoroalkylations
Abstract
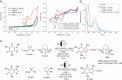
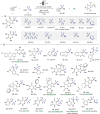
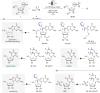
Chemo- and site-selective functionalization of complex molecules poses a fundamental challenge. Herein, we introduce a resource-economic photoelectrocatalysis strategy to enable versatile direct fluoroalkylations catalyzed by Earth-abundant iron and paired with the hydrogen evolution reaction (HER). Notably, the devised approach proved amenable to versatile late-stage C─H fluoroalkylations of bio-relevant heterocycles, such as xanthines, nucleobases, and nucleosides. Mechanistic studies supported a ligand-to-metal charge transfer-induced formation of the fluoroalkyl radical.
Keywords: C─H Functionalization; Electrophotochemistry; Fluoroalkylation; Iron catalysis; Sustainable chemistry.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
An Alternative Mechanism for C-C Desaturation Underscores a Dual-Controlled Mechanism for the Fate of Radical Intermediate in Iron(II)- and 2-(Oxo)glutarate-Dependent Oxygenase DfmD.J Am Chem Soc. 2025 Jun 18;147(24):20442-20455. doi: 10.1021/jacs.5c02361. Epub 2025 Jun 6. J Am Chem Soc. 2025. PMID: 40480968
-
Ultrafast solvent migration in an iron complex revealed by nonadiabatic dynamics simulations.Chem Sci. 2025 May 13;16(24):11128-11137. doi: 10.1039/d5sc01174d. eCollection 2025 Jun 18. Chem Sci. 2025. PMID: 40417306 Free PMC article.
-
Late-Stage Amination of Peptides on the Solid Phase.Chemistry. 2025 Jun 17;31(34):e202501229. doi: 10.1002/chem.202501229. Epub 2025 May 13. Chemistry. 2025. PMID: 40322874 Free PMC article.
-
Defining disease severity in atopic dermatitis and psoriasis for the application to biomarker research: an interdisciplinary perspective.Br J Dermatol. 2024 Jun 20;191(1):14-23. doi: 10.1093/bjd/ljae080. Br J Dermatol. 2024. PMID: 38419411 Free PMC article. Review.
-
Diamond Chemistry: Advances and Perspectives.Angew Chem Int Ed Engl. 2025 Jun 17;64(25):e202418683. doi: 10.1002/anie.202418683. Epub 2025 May 23. Angew Chem Int Ed Engl. 2025. PMID: 40208754 Free PMC article. Review.
References
-
- Cheng X., Lei A., Mei T.‐S., Xu H.‐C., Xu K., Zeng C., CCS Chem 2022, 4, 1120–1152.
-
- Huang H., Steiniger K. A., Lambert T. H., J. Am. Chem. Soc. 2022, 144, 12567–12583. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

