Microscale Delivery Systems for Hydrophilic Active Ingredients in Functional Consumer Goods
- PMID: 40223375
- PMCID: PMC11994985
- DOI: 10.1002/wnan.70009
Microscale Delivery Systems for Hydrophilic Active Ingredients in Functional Consumer Goods
Abstract
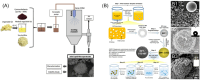
Hydrophilic active ingredients play a crucial role in formulated consumer products, encompassing antioxidants, flavoring substances, and pharmaceuticals. Yet, their susceptibility to environmental factors, such as light, pH, temperature, and humidity, poses challenges to their stability and sustained release. Microencapsulation offers a promising avenue to address these challenges, facilitating stabilization, targeted delivery, and enhanced efficacy of hydrophilic actives. However, despite significant advancements in the field, microencapsulation of hydrophilic actives remains at the forefront of innovation. This is primarily due to the intrinsic characteristics of hydrophilic actives, including small molecular weight and thus high permeability through many microcarriers (e.g., shells), which often necessitate complex and costly technologies to be developed. Moreover, in light of escalating regulatory frameworks, the pursuit of biodegradable and other compliant materials suitable for the entrapment of hydrophilic ingredients is gaining momentum. These advancements aim to provide alternatives to currently used non-degradable synthetic polymer materials. Research is currently pushing towards meeting these regulatory constraints via cutting-edge technologies to engineer novel microscale delivery systems for hydrophilic active ingredients, including microcapsules, microspheres, microneedles, and micropatches. Although still in its infancy, this approach holds true potential for revolutionizing the future of formulated consumer goods.
Keywords: drug delivery; hydrophilic active ingredients; microcapsules; micromanipulation; microneedles; micropatches; microspheres; microsponges.
© 2025 The Author(s). WIREs Nanomedicine and Nanobiotechnology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Ethosomes and Transfersomes: Principles, Perspectives and Practices.Curr Drug Deliv. 2017;14(5):613-633. doi: 10.2174/1567201813666160520114436. Curr Drug Deliv. 2017. PMID: 27199229
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Abdekhodaie, M. J. , Cheng J., and Wu X. Y.. 2015. “Effect of Formulation Factors on the Bioactivity of Glucose Oxidase Encapsulated Chitosan–Alginate Microspheres: In Vitro Investigation and Mathematical Model Prediction.” Chemical Engineering Science 125: 4–12. 10.1016/j.ces.2014.11.010. - DOI
-
- Abd‐Elal, R. M. A. , Elosaily G. H., Gad S., Khafagy E.‐S., and Mostafa Y.. 2020. “Full Factorial Design, Optimization, In Vitro and Ex Vivo Studies of Ocular Timolol‐Loaded Microsponges.” Journal of Pharmaceutical Innovation 15, no. 4: 651–663. 10.1007/s12247-019-09418-z. - DOI
-
- Adepu, S. , and Ramakrishna S.. 2021. “Controlled Drug Delivery Systems: Current Status and Future Directions.” Molecules 26, no. 19: 5905. https://www.mdpi.com/1420‐3049/26/19/5905. - PMC - PubMed
-
- Aldawood, F. K. , Andar A., and Desai S.. 2021. “A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications.” Polymers 13, no. 16: 2815. https://www.mdpi.com/2073‐4360/13/16/2815. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

