ADAPT NXT: Fixed Cycles or Every-Other-Week IV Efgartigimod in Generalized Myasthenia Gravis
- PMID: 40223516
- PMCID: PMC12172114
- DOI: 10.1002/acn3.70051
ADAPT NXT: Fixed Cycles or Every-Other-Week IV Efgartigimod in Generalized Myasthenia Gravis
Abstract
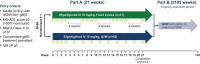
Objective: This phase 3b, open-label, randomized ADAPT NXT study investigated the efficacy, safety, and tolerability of efgartigimod administered in either a fixed cycles dosing regimen (3 cycles of 4 once-weekly infusions, with 4 weeks between cycles) or a cycle followed by every-other-week (Q2W) dosing.
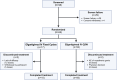
Methods: Adult participants with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis (gMG) were randomized 3:1 to Q2W or fixed cycles dosing of efgartigimod (10 mg/kg intravenously) for 21 weeks. The primary endpoint was the mean change from baseline in total Myasthenia Gravis Activities of Daily Living (MG-ADL) score averaged across 21 weeks.
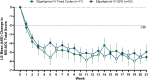
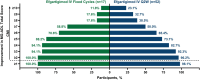
Results: Sixty-nine participants were treated (fixed cycles, n = 17; Q2W, n = 52). Least squares (LS) mean (95% CI) of the change from baseline in MG-ADL total score from Weeks 1 to 21 was -5.1 (-6.5 to -3.8) in the fixed cycles arm and -4.6 (-5.4 to -3.8) in the Q2W arm. Clinical improvements were observed in MG-ADL total scores as early as Week 1 and were maintained throughout the study. Achievement of minimal symptom expression (MG-ADL: 0-1) from Weeks 1 to 21 occurred in 47.1% (n = 8/17) and 44.2% (n = 23/52) of participants in the fixed cycles and Q2W arms, respectively. Efgartigimod was well tolerated; COVID-19, headache, and upper respiratory tract infection were the most common treatment-emergent adverse events.
Interpretation: Efgartigimod administered as either fixed cycles or Q2W dosing results in rapid, robust, and sustained clinically meaningful improvement. These results build upon previous studies and provide additional efgartigimod dosing approaches to achieve and sustain clinical efficacy in patients with gMG.
Keywords: efgartigimod; generalized myasthenia gravis; myasthenia gravis activities of daily living; neonatal Fc receptor.
© 2025 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
A.A.H. has received research support from argenx, Alexion Pharmaceuticals Inc., VielaBio, UCB Pharma, Genentech, Regeneron, and Sanofi. He has received consulting fees from argenx, Alexion Pharmaceuticals Inc., and UCB. K.G.C. has received consulting fees for advisory boards and/or received speaker honoraria from Alexion Pharmaceuticals Inc., Alnylam, Amicus, argenx, Biogen, CSL Behring, Ipsen, Janssen, Lupin, Pfizer, Roche, Sanofi‐Genzyme, and UCB. K.G.C. is the chairholder of the Emil von Behring Chair for Neuromuscular and Neurodegenerative Disorders by CSL Behring. V.B. has received research support from AZ‐Alexion, Grifols, CSL, UCB, argenx, Takeda, Octapharma, Akcea, Momenta (J&J), Immunovant, Ionis, and Viela. Y.H. has no disclosures to report. K.G. has received consulting/speaking honoraria from Alexion Pharmaceuticals Inc., and argenx, and consulting honoraria from UCB and Amgen. G.S. has received consulting fees/honoraria or support for meeting participation from Alexion Pharmaceuticals Inc., argenx, UCB, Immunovant Inc., and Biogen Inc. E.C.‐V. has received consulting/speaker fees from argenx, UCB, Alexion Pharmaceuticals Inc., and Janssen. E.B., D.G., A.S., R.H.J., D.H., and D.M. are employees of argenx. R.M. has received consulting fees/honoraria or support for meeting participation from Alexion Pharmaceuticals Inc., argenx, Ra Pharmaceuticals, Biomarin, Catalyst, UCB, TEVA, Merck, Roche, Janssen, and Biogen Inc. A.M. received speaker honoraria from Alexion Pharmaceuticals Inc., argenx, Grifols, SA, and Hormosan Pharma GmbH; honoraria from argenx, Alexion Pharmaceuticals Inc., UCB, Janssen, and Merck for consulting services; and financial research support (paid to his institution) from Octapharma, argenx, and Alexion Pharmaceuticals Inc. He is a member of the medical advisory board of the German Myasthenia Gravis Society. S.A. received speaker honoraria from Alexion Pharmaceuticals Inc., argenx, Sanofi, Pfizer, and LFB and honoraria from Alexion Pharmaceuticals Inc., UCB, Janssen, Sanofi, Pfizer, Biogen Inc., and LFB for consulting services.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
