Relationship Between Remote, Ambulatory Pulmonary Artery Pressures, and All-Cause Mortality in Patients With Chronic Heart Failure
- PMID: 40223608
- PMCID: PMC12165488
- DOI: 10.1161/CIRCHEARTFAILURE.124.012754
Relationship Between Remote, Ambulatory Pulmonary Artery Pressures, and All-Cause Mortality in Patients With Chronic Heart Failure
Abstract
Background: Hemodynamically guided management of patients with chronic heart failure (HF), using a remote, ambulatory pulmonary artery (PA) pressure monitor, has been shown to reduce mortality and morbidity. These improved outcomes were associated with a reduction in PA pressure. However, several pivotal questions remain unanswered: do systolic, diastolic, or mean PA pressures each predict all-cause mortality? Do PA pressures predict mortality across the ejection fraction (EF) spectrum? Do increases or decreases in PA pressure over time predict increases or decreases in mortality?
Methods: Retrospective analyses of data from CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients; n=550), GUIDE-HF (Hemodynamic-GUIDEed management of Heart Failure; n=2358), US PAS (CardioMEMS HF System Post Approval Study; n=1200), and MEMS-HF (CardioMEMS Monitoring Study for Heart Failure; n=234) were performed, including all enrolled patients regardless of treatment assignments (Total N=4342). PA systolic, PA diastolic, and PA mean pressures were examined in patients with HF and reduced EF (<50%, n=2562) and preserved EF (≥50%, n=1454). Baseline pressure (averaged over 14 days after implantation) and change in pressure (increase/decrease/no change) from baseline to 6 months (averaged over 14 days just before the 6-month time point) were related to all-cause mortality over a 2-year follow-up period.
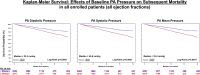
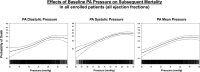
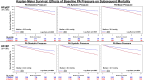
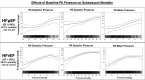
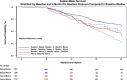
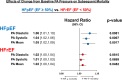
Results: Baseline PA diastolic, independent of other covariates, was a significant predictor of mortality (hazard ratio, 1.04 [95% CI, 1.03-1.05]; P<0.0001). Change in PA diastolic from baseline to 6 months (assessed as a continuous variable) was an independent predictor of mortality after 6 months (landmark analysis; hazard ratio, 1.03 [95% CI, 1.01-1.05]; P=0.0042). Change in PA diastolic from baseline to 6 months(assessed as a categorical variable) decrease or increase of >2 mm Hg compared with no change predicted a 14.7% decrease and 26.7% increase in mortality, respectively (P=0.0237). PA systolic and PA mean pressures in both HF with reduced EF and HF with preserved EF patients, for both baseline and change from baseline to 6 months, were also predictive of all-cause mortality.
Conclusions: Baseline PAP (systolic, diastolic, and mean) and change in PAP (systolic, diastolic, and mean) from baseline to 6 months were independent predictors of 2-year mortality in patients with chronic HF in both preserved and reduced EF.
Registration: URL: https://www.clinicaltrials.gov; Unique identifiers: CHAMPION, NCT00531661; GUIDE-HF, NCT03387813; USPAS, NCT02279888; MEMS-HF, NCT02693691.
Keywords: heart failure; humans; morbidity; pulmonary artery; stroke volume.
Conflict of interest statement
Dr Zile is a consultant for Eli Lily, Medtronic, and Novartis. Dr Abraham is a consultant for Abbott Vascular, AquaPass, CVRx, Impulse Dynamics, Medtronic, Sensible Medical Innovations, V-Wave, and Zoll Respicardia. Dr Stevenson is a consultant for Abbott Laboratories and Endotronix. Dr Costanzo is a consultant for Abbott Laboratories, is employed by Midwest Cardiovascular Institute, and is on the board of directors for Nuwelis. Dr Angermann is a consultant for Abbott Fund, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Vifor Pharma. Dr Mehra is a consultant for Abbott Laboratories, Broadview Ventures, Cadrenal, FIRE-1, Natera, Paragonix, and Second Heart Assist, has stock options in FineHeart, Leviticus, NupulseCV, and Transmedics, and is on the end-point review committee for Moderna. Dr Desai is a consultant for Abbott Pharmaceuticals, Alnylam Pharmaceuticals, AstraZeneca, Avidity Biopharma, Axon Therapies, Biofourmis, Boston Scientific, CVS Caremark, DTX Plus, Endotronix, GlaxoSmithKline, iRhythm Tehnologies, Medpace, Medtronic, Merck, New Amsterdam, Novartis, Parexel, Regeneron Pharmaceuticals, Relypsa, River2Renal, Roche Diagnostics, SCPharma, Verily, Veristat, and Zydus Pharmaceuticals and is on the end-point review committee for Baim Institute for Clinical Research and Stanford Center for Clinical Research. Dr Ducharme is a consultant for Abbott, AstraZeneca, and Novo Nordisk. Dr Johnson is employed by Abbott Laboratories. J. Lindefeld is a consultant for Abbott, AstraZeneca, Boehringer Ingelheim, CVRx, Edwards Lifesciences, and V-Wave. J. Henderson has nothing to disclose.
Figures
References
-
- Abraham WT, Stevenson LW, Bourge RC, Lindenfeld J, Bauman JG, Adamson PB. CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0 - PubMed
-
- Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, Krim SR, Maisel A, Mehra MR, Paul S, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398:991–1001. doi: 10.1016/S0140-6736(21)01754-2 - PubMed
-
- Lindenfeld J, Abraham WT, Maisel A, Zile M, Smart F, Costanzo MR, Mehra MR, Ducharme A, Sears SF, Desai AS, et al. Hemodynamic-GUIDEd management of Heart FAILURE (GUIDE-HF). Am Heart J. 2019;214:18–27. doi: 10.1016/j.ahj.2019.04.014 - PubMed
-
- Mehra MR. Primary results of the prospective single arm trial of hemodynamic-guided management of heart failure (GUIDE-HF). J Card Fail. 2024;30:312. doi: 10.1016/j.cardfail.2023.10.466
-
- Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD, Heywood JT, Jermyn RA, Pelzel J, Jonsson OT, et al. ; CardioMEMS Post-Approval Study Investigators. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS post-approval study. Circ Heart Fail. 2020;13:e006863. doi: 10.1161/CIRCHEARTFAILURE.119.006863 - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

