Activated immune infiltrates expand opportunities for targeted therapy in p53-abnormal endometrial carcinoma
- PMID: 40223796
- PMCID: PMC12146821
- DOI: 10.1002/path.6429
Activated immune infiltrates expand opportunities for targeted therapy in p53-abnormal endometrial carcinoma
Abstract
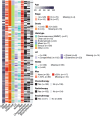
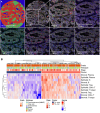
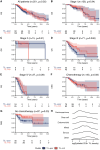
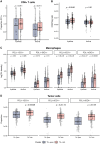
Tumor protein p53 mutated/abnormal (p53abn) endometrial carcinomas account for over 50% of deaths but comprise only 15% of all endometrial carcinomas. Most patients show limited response to standard-of-care chemotherapy with or without radiotherapy, and only a minority of cases are amenable to targeted therapies like poly-ADP ribose polymerase (PARP) inhibitors and HER2-directed therapies. Recent immunotherapy clinical trials have demonstrated remarkable efficacy, not only in mismatch repair deficient (MMRd) tumors but also in a subset of mismatch repair-proficient (MMRp) tumors. However, the immune microenvironment and its relationship to other therapeutic targets in MMRp endometrial carcinoma remains poorly understood. Here, we characterize the immune microenvironment of p53abn endometrial carcinoma, the most clinically aggressive subtype of MMRp endometrial carcinoma, and correlate antitumor immune signatures with other targetable alterations. We accrued 256 treatment-naïve p53abn endometrial carcinomas and systemically profiled T-cell, B-cell, myeloid, and tumor-cell populations with multiplex immunofluorescence to assess the tissue localization and functional status of immune cells. Shallow whole-genome sequencing was performed on a subset of 126 cases. Patterns of immune infiltration were compared to survival outcomes and mutational signatures. Mixture modeling divided p53abn endometrial carcinoma into tumor-infiltrating lymphocyte (TIL)-rich and TIL-poor subsets. Over 50% of tumors were TIL-rich. TIL-rich cases overexpressed targetable immune evasion molecules and were associated with longer overall and disease-specific survival in multivariate analysis. This effect was particularly pronounced in advanced stage disease and in patients who did not receive adjuvant chemotherapy. TIL did not associate with homologous recombination deficient mutational signatures or HER2 amplification. Our findings demonstrate a biological rationale for immunotherapy in a substantial subset of patients with p53abn endometrial cancer and may help inform combination therapies with immune checkpoint inhibition, PARP inhibitors, and anti-HER2 agents. © 2025 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: HER2; PARP inhibitors; endometrial cancer; immunotherapy; p53abn; tumor microenvironment; tumor‐infiltrating lymphocytes.
© 2025 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Figures
References
-
- Siegel RL, Giaquinto AN, Ahmedin J. Cancer statistics, 2024. CA Cancer J Clin 2024; 74: 12–49. - PubMed
-
- Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics‐based clinical classifier for endometrial cancer. Cancer 2017; 123: 802–813. - PubMed
-
- Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population‐based case series. Ann Oncol 2018; 29: 1180–1188. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

