Novel N-(4,5,6,7-tetrahydrobenzisoxazol-4-yl)amides as HSP90 inhibitors: design, synthesis and biological evaluation
- PMID: 40223823
- PMCID: PMC11987037
- DOI: 10.1039/d4md00904e
Novel N-(4,5,6,7-tetrahydrobenzisoxazol-4-yl)amides as HSP90 inhibitors: design, synthesis and biological evaluation
Abstract
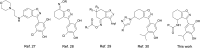
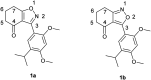
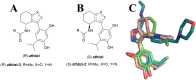
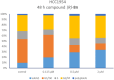
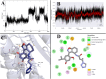
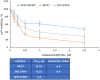
Novel N-(4,5,6,7-tetrahydrobenzisoxazol-4-yl)amide derivatives were designed and synthesized as potential HSP90 inhibitors. The synthetic pathway commenced with 6,7-dihydrobenzo[d]isoxazol-4(5H)-ones, utilizing the Ritter reaction as a key step. Molecular docking, molecular dynamics simulations, and MM/GBSA analysis guided the selection of compounds for synthesis and provided insights into the interaction mode of the most active compound with HSP90α. The synthesized compounds exhibited significant antiproliferative effects against breast cancer cell lines ERα+ MCF7 and HER2+ HCC1954. Lead compounds with submicromolar IC50 values, initially synthesized as racemates, were subsequently obtained and tested in their enantiopure forms. In HER2+ HCC1954 cancer cells, the molecular pathways regulated by compound (R)-8n were characterized. Treatment with compound (R)-8n resulted in the pronounced suppression of HSP90-related pathways, including key oncoreceptors (HER2, EGFR, c-MET) and mitogenic kinases (AKT, CDK4). Additionally, compound (R)-8n induced apoptosis, as evidenced by the accumulation of cleaved PARP. The inhibitory effect of compound (R)-8n on the HSP90 pathway was corroborated by molecular modeling and further validated through the observed suppression of client proteins, along with an upregulation of HSP70, a well-established marker of HSP90 inhibition. The activity of compound (R)-8n was associated with cell cycle arrest at the G2/M phases, ultimately leading to dose-dependent cell death. Notably, compound (R)-8n demonstrated substantial selectivity toward breast tumor cells. These findings suggest that N-(4,5,6,7-tetrahydrobenzisoxazol-4-yl)amides represent a promising class of HSP90 inhibitors for anticancer therapy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

