Diuretics: a review of the pharmacology and effects on glucose homeostasis
- PMID: 40223924
- PMCID: PMC11985539
- DOI: 10.3389/fphar.2025.1513125
Diuretics: a review of the pharmacology and effects on glucose homeostasis
Abstract
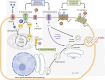
Thiazides, thiazide-like and loop diuretics are commonly prescribed to manage hypertension and heart failure. The main mechanism of action of these diuretics involve inhibition of Na+ reabsorption in the kidneys, leading to increased urine production. While effective, diuretics, particularly hydrochlorothiazide, have been linked to altered glucose metabolism and other metabolic issues. These disruptions in fuel homeostasis are not clearly related to their primary action of fluid management, raising concerns for patients with metabolic syndrome, in which high blood pressure coexists with obesity, insulin resistance, glucose intolerance and dyslipidemia. In this review, we conducted an extensive examination of existing literature on these classes of diuretics, covering publications from the late 1950s to the present. Our objective was to investigate the origins, development and current understanding of the widely recognized association between the use of diuretics in general and their potential negative impact on glucose homeostasis. We focused on the clinical and experimental evidence of the most commonly prescribed diuretics: hydrochlorothiazide, chlorthalidone, bumetanide and furosemide. On one hand, the clinical evidence supports the hypothesis that the metabolic effects on glucose homeostasis are primarily linked to hydrochlorothiazide, with little, if any impact observed in other diuretics. In addition, these metabolic effects do not appear to be related to their diuretic action or intended pharmacological targets, raising concerns about the long-term metabolic impact of specific diuretics, particularly in vulnerable populations, including those with metabolic syndrome. On the other hand, the experimental evidence using animal models suggest variable effects of diuretics in insulin secretion and general glucose metabolism. Although the mechanisms involved are not clearly understood, further research is needed to uncover the molecular mechanisms by which certain diuretics disrupt fuel metabolism and contribute to metabolic disturbances.
Keywords: diabetes; hyperglycemia; hypertension; insulin; loop diuretics; metabolic syndrome; overweight; thiazides.
Copyright © 2025 Di Fulvio, Rathod and Khader.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adem S., Ciftci M. (2016). Purification and characterization of glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and glutathione reductase from rat heart and inhibition effects of furosemide, digoxin, and dopamine on the enzymes activities. J. Biochem. Mol. Toxicol. 30, 295–301. 10.1002/jbt.21793 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

