CCL7 promotes macrophage polarization and synovitis to exacerbate rheumatoid arthritis
- PMID: 40224025
- PMCID: PMC11987677
- DOI: 10.1016/j.isci.2025.112177
CCL7 promotes macrophage polarization and synovitis to exacerbate rheumatoid arthritis
Abstract
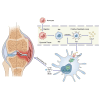
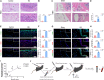
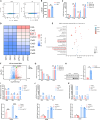
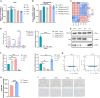
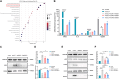
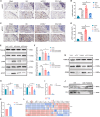
Chemokine C-C motif ligand 7 (CCL7) is implicated in various immune and inflammatory processes; however, its role in rheumatoid arthritis (RA) remains unclear. In this study, we observed that CCL7 expression was upregulated in synovial M1-polarized macrophages and in the serum of RA mice and patients. CCL7 was found to promote macrophage polarization toward the M1 phenotype while inhibiting M2 differentiation in vitro. Furthermore, intra-articular injection of recombinant CCL7 protein in mice resulted in enhanced M1 polarization, increased inflammation, and fibrosis within synovial tissues, which exacerbated arthritis-associated pain. These effects were partially mitigated by treatment with a CCL7 neutralizing antibody. Mechanistically, we identified a CCL7 autocrine positive feedback loop that amplifies inflammation via the CCL7-CCR1-JAK2/STAT1 pathway. Collectively, our findings reveal a previously unrecognized CCL7-mediated autocrine inflammatory amplification loop that modulates macrophage polarization and exacerbates RA progression, positioning CCL7 as a potential therapeutic target for RA.
Keywords: biological sciences; immune system disorder; immunology; natural sciences.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Endothelial-Derived CCL7 Promotes Macrophage Polarization and Aggravates Septic Acute Lung Injury via CCR1-Mediated STAT1 Succinylation.Adv Sci (Weinh). 2025 Aug 4:e06209. doi: 10.1002/advs.202506209. Online ahead of print. Adv Sci (Weinh). 2025. PMID: 40755420
-
Suberosin attenuates rheumatoid arthritis by repolarizing macrophages and inhibiting synovitis via the JAK/STAT signaling pathway.Arthritis Res Ther. 2025 Jan 21;27(1):12. doi: 10.1186/s13075-025-03481-3. Arthritis Res Ther. 2025. PMID: 39838477 Free PMC article.
-
CCL7 contributes to angiotensin II-induced abdominal aortic aneurysm by promoting macrophage infiltration and pro-inflammatory phenotype.J Cell Mol Med. 2021 Aug;25(15):7280-7293. doi: 10.1111/jcmm.16757. Epub 2021 Jun 29. J Cell Mol Med. 2021. PMID: 34189838 Free PMC article.
-
Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review.Autoimmun Rev. 2019 Nov;18(11):102397. doi: 10.1016/j.autrev.2019.102397. Epub 2019 Sep 11. Autoimmun Rev. 2019. PMID: 31520798
-
The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis.Front Immunol. 2022 May 19;13:867260. doi: 10.3389/fimmu.2022.867260. eCollection 2022. Front Immunol. 2022. PMID: 35663975 Free PMC article. Review.
References
-
- Liang W., Feng R., Li X., Duan X., Feng S., Chen J., Li Y., Chen J., Liu Z., Wang X., et al. A RANKL-UCHL1-sCD13 negative feedback loop limits osteoclastogenesis in subchondral bone to prevent osteoarthritis progression. Nat. Commun. 2024;15:8792. doi: 10.1038/s41467-024-53119-2. - DOI - PMC - PubMed
-
- Maeda K., Yoshida K., Nishizawa T., Otani K., Yamashita Y., Okabe H., Hadano Y., Kayama T., Kurosaka D., Saito M. Inflammation and Bone Metabolism in Rheumatoid Arthritis: Molecular Mechanisms of Joint Destruction and Pharmacological Treatments. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23052871. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

