Minimal residual disease assessment following CD19-targeted therapy in B-cell precursor acute lymphoblastic leukemia using standardized 12-color flow cytometry: A EuroFlow study
- PMID: 40224162
- PMCID: PMC11993931
- DOI: 10.1002/hem3.70125
Minimal residual disease assessment following CD19-targeted therapy in B-cell precursor acute lymphoblastic leukemia using standardized 12-color flow cytometry: A EuroFlow study
Abstract
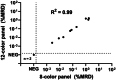
Detection of minimal/measurable residual disease (MRD) is a critical prognostic marker in B-cell precursor acute lymphoblastic leukemia (BCP-ALL). The EuroFlow Consortium previously developed an 8-color flow cytometric MRD protocol, effective for >98% of BCP-ALL patients treated with chemotherapy. This study aimed to enhance MRD detection, particularly for patients treated with CD19-targeted therapies, by expanding the EuroFlow protocol to a 12-color panel. This new panel incorporates additional B-cell markers and exclusion T/NK-cell markers (CD3 and CD7). Through an evaluation of 237 diagnostic BCP-ALL samples, CD22, CD24, and HLA-DR were selected as additional B-cell gating markers. Two 12-color tubes were technically optimized and clinically validated across 101 patient follow-up samples, demonstrating excellent concordance with molecular MRD levels (R 2 = 0.88). The 12-color BCP-ALL MRD tubes were compatible with the previously developed 8-color automated gating and identification (AGI) tool and demonstrated good reproducibility. Our findings indicate that the 12-color panel performs comparably to the 8-color BCP-ALL MRD panel, including both CD19-positive and CD19-negative cases. However, it offers an improved definition of the B-cell lineage, particularly for expert-guided manual data analysis, and provides additional information on the expression of the targetable marker CD22.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
J. J. M. v. D., A. O., and V. H. J. v. d. V. each report being one of the inventors on the EuroFlow‐owned patent PCT/NL2010/050332 (methods, reagents and kits for flow cytometric immunophenotyping of normal, reactive, and malignant leukocytes). The Infinicyt software is based on the intellectual property (IP) of some EuroFlow laboratories (University of Salamanca in Spain) and the scientific input of other EuroFlow members. All aforementioned intellectual property and related patents are licensed to Cytognos (Salamanca, ES) and BD Biosciences (San José, CA), companies that pay royalties to the EuroFlow Consortium. These royalties are exclusively used for the continuation of the EuroFlow collaboration and sustainability of the EuroFlow Consortium. V. H. J. v. d. V. reports a Laboratory Services Agreement with BD Biosciences, Cytognos, and Agilent; all related fees are for the Erasmus MC. J. J. M. v. D. and A. O. report an Educational Services Agreement from BD Biosciences (San José, CA) and a Scientific Advisor Agreement with Cytognos; all related fees and honoraria are for the involved university departments at Leiden University Medical Center and University of Salamanca. M. B. reports a Laboratory Services Agreement with BD Biosciences and Cytognos; all related fees are for the UKSH. M. B. received personal fees from Incyte (advisory board); financial support for reference diagnostics from Amgen and Celgene; grants and personal fees from Amgen (advisory board, speakers bureau, travel and support); and personal fees from Janssen and BD (speakers bureau), all outside the submitted work. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Minimal residual disease assessment in B-cell precursor acute lymphoblastic leukemia by semi-automated identification of normal hematopoietic cells: A EuroFlow study.Cytometry B Clin Cytom. 2024 Jul;106(4):252-263. doi: 10.1002/cyto.b.22143. Epub 2023 Sep 22. Cytometry B Clin Cytom. 2024. PMID: 37740440
-
How I Investigate Measurable Residual Disease in B-Cell Precursor Acute Lymphoblastic Leukemia After Therapy With Bi-Specific Monoclonal Antibodies and 19CAR-T Cells.Int J Lab Hematol. 2025 Jun;47(3):398-406. doi: 10.1111/ijlh.14448. Epub 2025 Feb 26. Int J Lab Hematol. 2025. PMID: 40007453 Review.
-
Flow cytometric minimal residual disease assessment in B-cell precursor acute lymphoblastic leukaemia patients treated with CD19-targeted therapies - a EuroFlow study.Br J Haematol. 2022 Apr;197(1):76-81. doi: 10.1111/bjh.17992. Epub 2021 Dec 8. Br J Haematol. 2022. PMID: 34881427 Free PMC article.
-
The Evolving Landscape of Flowcytometric Minimal Residual Disease Monitoring in B-Cell Precursor Acute Lymphoblastic Leukemia.Int J Mol Sci. 2024 Apr 30;25(9):4881. doi: 10.3390/ijms25094881. Int J Mol Sci. 2024. PMID: 38732101 Free PMC article. Review.
-
15-color highly sensitive flow cytometry assay for post anti-CD19 targeted therapy (anti-CD19-CAR-T and blinatumomab) measurable residual disease assessment in B-lymphoblastic leukemia/lymphoma: Real-world applicability and challenges.Eur J Haematol. 2024 Jan;112(1):122-136. doi: 10.1111/ejh.14102. Epub 2023 Sep 14. Eur J Haematol. 2024. PMID: 37706583
References
-
- Howlader NN, Krapcho AM, Miller M, et al., eds. SEER Cancer Statistics Review: 1975‐2013. 2016. https://seer.cancer.gov/archive/csr/1975_2013/
-
- Burkart M, Dinner S. Advances in the treatment of Philadelphia chromosome negative acute lymphoblastic leukemia. Blood Rev. 2024;66:101208. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
