Evolution and function of galectins in Xenopus laevis: Comparison with mammals and new perspectives
- PMID: 40224191
- PMCID: PMC11986560
- DOI: 10.1016/j.bbadva.2025.100157
Evolution and function of galectins in Xenopus laevis: Comparison with mammals and new perspectives
Abstract
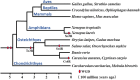
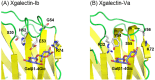
Galectins are metal-independent sugar-binding proteins that recognize galactose (the β-galactoside structure) and regulate the cross-linking of sugar chains between cells and the extracellular matrix. Their specificity for galactose is attributed to their highly conserved carbohydrate recognition domain. Galectins participate in biological processes across species, including development, differentiation, morphogenesis, tumor progression, metastasis, immunity, and apoptosis. However, the relationship between the binding of galectin to sugar chains (glycans) and their biological functions remains unclear. Thus, a comprehensive functional analysis of galectins is required to better characterize their evolutionarily conserved and unique functions. We have previously identified and characterized 12 Xenopus laevis galectins (xgalectins), the only non-mammalian vertebrate species in which galectins have been comprehensively characterized to date. In this review, we present the latest findings on the types and functions of xgalectins and discuss prospects for elucidating their diverse functions from an evolutionary perspective through comparisons with mammalian galectins.
Keywords: Carbohydrate recognition; Evolution; Galectins; Sequence conservation; Whole-genome duplication; Xenopus laevis.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- S. Ohno, Evolution by gene duplication (1970).
-
- Holland P.W., Garcia-Fernandez J., Williams N.A., Sidow A. Gene duplications and the origins of vertebrate development. Dev. Suppl. 1994:125–133. - PubMed
LinkOut - more resources
Full Text Sources

