Exploration of the plasma proteomic profile of patients at risk of thromboembolic events
- PMID: 40224277
- PMCID: PMC11986537
- DOI: 10.1016/j.rpth.2025.102713
Exploration of the plasma proteomic profile of patients at risk of thromboembolic events
Abstract
Background: The elevated health burden of thromboembolic events necessitates development of blood-based risk monitoring tools.
Objectives: We explored the potential of mass spectrometry-based plasma proteomics to provide insights into underlying plasma protein signatures associated with treatment and occurrence of thromboembolic events.
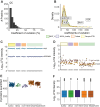
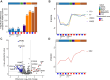
Methods: Utilizing a high-throughput, data-independent acquisition, discovery-based proteomics workflow, we analyzed 434 plasma proteomes from different groups of individuals with elevated risk of thromboembolic events, including individuals I) on vitamin K antagonists (VKAs; n = 130), II) with a prior venous thromboembolism (n = 10), III) with acute cerebral venous sinus thrombosis (n = 10, and IV) with SARS-CoV-2 infection (n = 67). Plasma protein levels measured with mass spectrometry were correlated with international normalized ratio and conventional clinical laboratory measurements. Plasma profile differences between different groups were assessed using principal component analysis, moderated t-test, and clustering analysis.
Results: Plasma protein levels were in agreement with conventional clinical laboratory parameters, including albumin and fibrinogen. Levels of vitamin K-dependent proteins inversely correlated with international normalized ratio. In the individual studies, we found decreased levels of vitamin K-dependent coagulation proteins in patients on VKAs, alterations in inflammatory signatures among CVST patients and a distinctive signature indicative of SARS-CoV-2 infection. However, no protein signature associated with a thromboembolic event could be identified neither in individual nor combined studies.
Conclusion: Although VKA treatment-specific and disease-specific signatures were captured, our study highlights that the challenges of discovering biomarkers in patients at risk of thromboembolic events lie in the heterogeneity of individual plasma profiles in relation to treatment and etiology.
Keywords: biomarkers; mass spectrometry; plasma; proteomics; thrombosis.
© 2025 The Authors.
Figures




References
-
- Raskob G.E., Angchaisuksiri P., Blanco A.N., Buller H., Gallus A., Hunt B.J., et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–2371. - PubMed
-
- Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The global burden of cardiovascular diseases and risk. J Am Coll Cardiol. 2022;80:2361–2371. - PubMed
-
- Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. - PubMed
-
- Timp J.F., Braekkan S.K., Lijfering W.M., van Hylckama Vlieg A., Hansen J.B., Rosendaal F.R., et al. Prediction of recurrent venous thrombosis in all patients with a first venous thrombotic event: the Leiden Thrombosis Recurrence Risk Prediction model (L-TRRiP) PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002883. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

