Safety and efficacy of immunoguided prophylaxis for cytomegalovirus disease in low-risk lung transplant recipients in Spain: a multicentre, open-label, randomised, phase 3, noninferiority trial
- PMID: 40224372
- PMCID: PMC11992424
- DOI: 10.1016/j.lanepe.2025.101268
Safety and efficacy of immunoguided prophylaxis for cytomegalovirus disease in low-risk lung transplant recipients in Spain: a multicentre, open-label, randomised, phase 3, noninferiority trial
Abstract
Background: The standard prophylaxis treatment for cytomegalovirus (CMV) disease in CMV-seropositive lung transplant recipients is six months of prophylaxis with valganciclovir followed by six months of pre-emptive therapy. This protocol is associated with adverse events and risk of resistance. We have previously shown that prophylaxis can be suspended in CMV-seropositive kidney transplant recipients receiving thymoglobulin without increasing the risk of CMV disease and reducing the incidence of neutropenia. The objective of the current study is to demonstrate that immunoguided prophylaxis is effective and safe in seropositive lung transplant recipients.
Methods: A phase III, multicentre, randomised, open-label, noninferiority clinical trial was conducted in adult lung transplant recipients. Patients were randomised (1:1) to two groups: (1) immunoguided prophylaxis (IP), consisting of 3 months of universal prophylaxis followed by CMV-specific cell-mediated immunity-guided discontinuation, or (2) standard prophylaxis (SP), consisting of 6 months of prophylaxis followed by pre-emptive therapy, both for a total of 12 months. The noninferiority margin was 7%. The primary and secondary efficacy endpoints were CMV disease and asymptomatic CMV replication at month 18. The primary and secondary safety endpoints were incidence of neutropenia (defined as neutrophil count <1500 cells/μL), incidence of rejection and number of days of valganciclovir prophylaxis. This trial was registered in EudraCT (2018-003300-39) and ClinicalTrials.gov (NCT03699254). This trial has been completed.
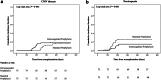
Findings: Patients were recruited between April 2019 and December 2021 in seven Spanish centres. A total of 150 patients were randomised (75 patients per group). Incidence of CMV disease at month 18 did not differ among groups (18·7% [14 patients] vs. 16·0% [12 patients]; risk difference [RD] -0·03 [95% CI -0·15% to 0·06%]; P = 0·620) but occurred earlier in the IP group compared to the SP group. The proportion of patients who developed CMV disease at ≤180 days after transplant was higher in the IP group compared with the SP group (8% [6 patients] vs. 0% [0 patients]; RD -0·08 [95% CI -0·14 to -0·02; P = 0·009]). Asymptomatic CMV replication was reduced in the IP group vs. the SP group (4·0% [3 patients] vs. 16·0% [12 patients]; adjusted RD 0·12 [95% CI 0·03-0·21; P = 0·009]). A total of 30 patients (40%) in the IP group did not require prophylaxis from month 4 to 12. No significant difference was observed in the proportion of patients with neutropenia during months 4 to 7 (14·7% [11 patients] vs. 25·3% [19 patients]; RD 0·11 [95% CI -0·02 to 0·23]; P = 0·090) or rejection (33·3% [25 patients] vs. 30·7% [23 patients]; RD -0·03 [95% CI -0·18 to 0·12; P = 0·690]). The median days of valganciclovir was lower in the IP group than in the SP group (137 [92-266] vs. 198 [173-281]; P < 0.001).
Interpretation: Immunoguided prophylaxis was noninferior to the standard of care in preventing CMV disease in lung transplant recipients. It could be considered for implementing in clinical practice in CMV-seropositive lung transplant recipients upon considering the study limitations.
Funding: Carlos III Health Institute, the SATOT Research Grant, the CIBER (Biomedical Network Research Centre Consortium), the Ministry of Science and Innovation, and the European Union.
Keywords: Clinical trial; Cytomegalovirus infection; IGRA-CMV; Immunoguided prophylaxis; Interferon-gamma; Lung transplantation.
© 2025 Published by Elsevier Ltd.
Conflict of interest statement
J T-C received research funding from the Carlos III Health Institute (grant numbers PI18/00099); a grant from Andalusian Society of Organ and Tissue Transplantation (SATOT); research funding from Biomedical Research Center for Infectious Diseases Network (CIBERIFEC); research funding and consulting honorary from Merck Sharp & Dohme (MSD); and honorary for educational activities from QIAGEN. A P-V, JM V-B, D I-F, R A, P U-G, A P and R F-M have participated in the project funded by Carlos III Health Institute (grant numbers PI18/00099). A P-V, R F-M, A C, JJ C, I M, B G-G and S C are staff collaborators at the Centre for Biomedical Research Network on Infectious Diseases (CIBERINFEC). S C and JJ C have also received honorarium for educational activities from QIAGEN. Moreover, S C has received support for attending educational activities and research support (material) from QIAGEN. V M and C B received support by the Centre for Biomedical Research Network on Respiratory Diseases (CIBERES). C B has also received honoraria for advisory boards from Chiesi, Janssen, and Astellas. V M has also received honoraria for Consulting from Biotest. E R-A has received funding from the Subprograma Rio Hortega, Instituto de Salud Carlos III, Spanish Government (CM23/00194). No other disclosures were reported.
Figures
References
-
- Monforte V., Sintes H., López-Gallo C., et al. Risk factors, survival, and impact of prophylaxis length in cytomegalovirus-seropositive lung transplant recipients: a prospective, observational, multicenter study. Transpl Infect Dis. 2017;19(3) - PubMed
-
- Torre-Cisneros J., Aguado J.M., Caston J.J., et al. Management of cytomegalovirus infection in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant Rev. 2016;30(3):119–143. - PubMed
-
- Kotton C.N., Kumar D., Caliendo A.M., et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102:900–931. - PubMed
-
- Martín-Gandul C., Pérez-Romero P., González-Roncero F.M., et al. Clinical impact of neutropenia related with the preemptive therapy of CMV infection in solid organ transplant recipients. J Infect. 2014;69(5):500–506. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous