Integrated Network Pharmacology, Molecular Modeling, LC-MS Profiling, and Semisynthetic Approach for the Roots of Rubia tinctorum L. Metabolites in Cancer Treatment
- PMID: 40224436
- PMCID: PMC11983213
- DOI: 10.1021/acsomega.4c09853
Integrated Network Pharmacology, Molecular Modeling, LC-MS Profiling, and Semisynthetic Approach for the Roots of Rubia tinctorum L. Metabolites in Cancer Treatment
Abstract
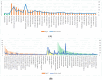
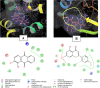
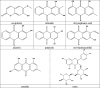
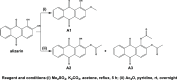
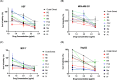
Rubia tinctorum L. is one of the most widely used plants in folk medicine, with many reported pharmacological activities. One of these valuable activities is its anticancer efficacy. The aim of this study is to explore the multilevel mechanisms of R. tinctorum metabolites in cancer treatment using network pharmacology, together with molecular docking and in vitro studies. The network pharmacology analysis enabled us to reveal the hit anticancer R. tinctorum constituents, which were found to be acacetin, alizarin, anthragallol, 2-hydroxyanthraquinone, and xanthopurpurin. The most enriched cancer-linked target genes were PLCG1, BCL2, CYP1B1, NSD2, and ESR2. The pathways that were mostly involved in the anticancer mechanism of R. tinctorum metabolites were found to be metabolic pathways as well as pathways in cancer and apoptosis. Molecular docking of the identified hit anticancer constituents on the active sites of the most enriched genes unveiled that acacetin and alizarin possessed the lowest binding energies on the active sites of NSD2 and BCL2, respectively. While anthragallol showed the most stabilized interaction on the active sites of PLCG1, CYP1B1, and ESR2. Consequently, R. tinctorum extracts were evaluated for their in vitro cytotoxicity on a panel of cancerous cells. Among the tested R. tinctorum extracts, the chloroform extract was the strongest one with an IC50 = 3.987 μg/mL on the MCF-7 breast cancer cell line. Consequently, it was subjected to chromatographic separation and purification to isolate its major components with reported anticancer activity (scopoletin, rubiadin, chrysophanic acid, alizarin, purpurin, nor-damnacanthal, emodin, and rutin). Alizarin and purpurin constituted the main anthraquinones in R. tinctorum . Thus, they were quantified using LC/MS analysis. Moreover, a semisynthetic approach of alizarin toward the enhancement of its anticancer effect on the tested cancer cells was attained. Among the synthesized compounds, 2-methyl alizarin was the most active one with an IC50 = 8.878 μg/mL against the HepG2 cell line. This study provides deep insights into the anticancer mechanisms of R. tinctorum metabolites for the first time using network pharmacology and valorizes their significance as valuable anticancer agents.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Targeted tumor therapy by Rubia tinctorum L.: analytical characterization of hydroxyanthraquinones and investigation of their selective cytotoxic, adhesion and migration modulator effects on melanoma cell lines (A2058 and HT168-M1).Cancer Cell Int. 2015 Dec 18;15:119. doi: 10.1186/s12935-015-0271-4. eCollection 2015. Cancer Cell Int. 2015. PMID: 26690297 Free PMC article.
-
Isolation and extraction of lucidin primeveroside from Rubia tinctorum L. and crystal structure elucidation.Phytochemistry. 2013 Nov;95:105-8. doi: 10.1016/j.phytochem.2013.07.001. Epub 2013 Jul 25. Phytochemistry. 2013. PMID: 23891215
-
Alizarin and Purpurin from Rubia tinctorum L. Suppress Insulin Fibrillation and Reduce the Amyloid-Induced Cytotoxicity.ACS Chem Neurosci. 2021 Jun 16;12(12):2182-2193. doi: 10.1021/acschemneuro.1c00177. Epub 2021 May 25. ACS Chem Neurosci. 2021. PMID: 34033711
-
Anthraquinone Production from Cell and Organ Cultures of Rubia Species: An Overview.Metabolites. 2022 Dec 26;13(1):39. doi: 10.3390/metabo13010039. Metabolites. 2022. PMID: 36676964 Free PMC article. Review.
-
Chemistry, Biosynthesis, Physicochemical and Biological Properties of Rubiadin: A Promising Natural Anthraquinone for New Drug Discovery and Development.Drug Des Devel Ther. 2021 Nov 3;15:4527-4549. doi: 10.2147/DDDT.S338548. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34764636 Free PMC article. Review.
References
-
- Hassanpour S. H.; Dehghani M. Review of Cancer from Perspective of Molecular. J. Cancer Res. Pract. 2017, 4, 127–129. 10.1016/j.jcrpr.2017.07.001. - DOI
-
- Feron O.; Abalo R.; Nurgali K.; Jagoe R. T. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Editorial on the Research Topic Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae?. Front. Pharmacol. 2018, 1, 245.10.3389/fphar.2018.00245. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
