Review of Competitive Adsorption of CO2/CH4 in Shale: Implications for CO2 Sequestration and Enhancing Shale Gas Recovery
- PMID: 40224438
- PMCID: PMC11983224
- DOI: 10.1021/acsomega.4c08678
Review of Competitive Adsorption of CO2/CH4 in Shale: Implications for CO2 Sequestration and Enhancing Shale Gas Recovery
Abstract
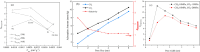
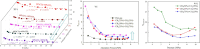
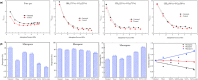
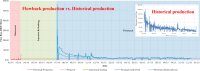
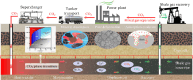
The injection of CO2 into shale gas reservoirs can not only enhance shale gas recovery (ESGR), but also realize CO2 geological storage (CGS). In this study, the competitive adsorption behaviors of CO2 and CH4 in shale were systematically reviewed, and the implication for shale gas recovery efficiency and CO2 storage potential were discussed. The adsorption advantage of shale for CO2 compared to CH4 provides a guarantee of the feasibility of supercritical CO2 (ScCO2) enhanced shale gas exploitation technology. The selective adsorption coefficient of CO2 and CH4 by shale (S CO2/CH4 ) is an important parameter in evaluating the competitive adsorption behavior of CO2/CH4 in shale gas reservoirs, which is closely related to the mineral composition, reservoir temperature, pressure conditions, water content, and mixed gas composition ratio. In addition, the injection type, injection mode, and injection rate of gases also exhibit different effects on CO2/CH4 competitive adsorption. Furthermore, the interaction between ScCO2 and the water-rock system will change the mineral composition and microstructure of shale, which will lead to changes in the adsorption behavior of shale on CO2 and CH4, so its influence on the competitive adsorption of CO2/CH4 cannot be ignored. Future research should integrate different research methods and combine with practical engineering to reveal the competitive adsorption mechanism of CO2/CH4 in shale reservoirs from both micro and macro aspects. This study can provide support for the integration technology of ScCO2 enhanced shale gas exploitation and its geological storage.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











Similar articles
-
Study of CO2 Enhancing Shale Gas Recovery Based on Competitive Adsorption Theory.ACS Omega. 2020 Sep 2;5(36):23429-23436. doi: 10.1021/acsomega.0c03383. eCollection 2020 Sep 15. ACS Omega. 2020. PMID: 32954196 Free PMC article.
-
CO2/CH4 Competitive Adsorption in Shale: Implications for Enhancement in Gas Production and Reduction in Carbon Emissions.Environ Sci Technol. 2019 Aug 6;53(15):9328-9336. doi: 10.1021/acs.est.9b02432. Epub 2019 Jul 29. Environ Sci Technol. 2019. PMID: 31318200
-
Molecular insights into competitive adsorption of CO2/CH4 mixture in shale nanopores.RSC Adv. 2018 Oct 3;8(59):33939-33946. doi: 10.1039/c8ra07486k. eCollection 2018 Sep 28. RSC Adv. 2018. PMID: 35548842 Free PMC article.
-
Comprehensive Review of CO2 Adsorption on Shale Formations: Exploring Widely Adopted Isothermal Models and Calculation Techniques.ACS Omega. 2024 Dec 13;9(51):50078-50096. doi: 10.1021/acsomega.4c04324. eCollection 2024 Dec 24. ACS Omega. 2024. PMID: 39741837 Free PMC article. Review.
-
Different ways to approach shale reservoirs' CO2 storage potential in America.Heliyon. 2023 Jul 20;9(8):e18458. doi: 10.1016/j.heliyon.2023.e18458. eCollection 2023 Aug. Heliyon. 2023. PMID: 37560675 Free PMC article. Review.
References
-
- Zhang C.-Y.; Chai X.-S.; Xiao X.-M. A simple method for correcting for the presence of minor gases when determining the adsorbed methane content in shale. Fuel 2015, 150, 334–338. 10.1016/j.fuel.2015.02.050. - DOI
-
- Zhang F.; Damjanac B.; Maxwell S. Investigating Hydraulic Fracturing Complexity in Naturally Fractured Rock Masses Using Fully Coupled Multiscale Numerical Modeling. Rock Mechanics and Rock Engineering 2019, 52 (12), 5137–5160. 10.1007/s00603-019-01851-3. - DOI
-
- Zhang X.; Lu Y.; Tang J.; Zhou Z.; Liao Y. Experimental study on fracture initiation and propagation in shale using supercritical carbon dioxide fracturing. Fuel 2017, 190, 370–378. 10.1016/j.fuel.2016.10.120. - DOI
-
- Middleton R. S.; Gupta R.; Hyman J. D.; Viswanathan H. S. The shale gas revolution: Barriers, sustainability, and emerging opportunities. Applied Energy 2017, 199, 88–95. 10.1016/j.apenergy.2017.04.034. - DOI
-
- Yang R.; Liu X.; Yu R.; Hu Z.; Duan X. Long short-term memory suggests a model for predicting shale gas production. Applied Energy 2022, 322, 119415.10.1016/j.apenergy.2022.119415. - DOI
Publication types
LinkOut - more resources
Full Text Sources
