Design and Computational Study of Sulfonamide-Modified Cannabinoids as Selective COX-2 Inhibitors Using Semiempirical Quantum Mechanical Methods: Drug-like Properties and Binding Affinity Insights
- PMID: 40224452
- PMCID: PMC11983223
- DOI: 10.1021/acsomega.5c00562
Design and Computational Study of Sulfonamide-Modified Cannabinoids as Selective COX-2 Inhibitors Using Semiempirical Quantum Mechanical Methods: Drug-like Properties and Binding Affinity Insights
Abstract
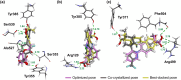
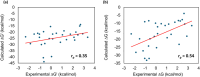
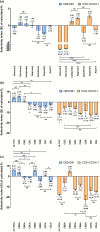
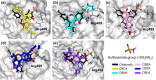
Cyclooxygenase (COX) is one of the concerned targets in the development of anti-inflammatory therapies. Using semiempirical quantum mechanical (SQM) methods with implicit solvation, we investigated the binding free energies and selectivity of natural cannabinoids and their sulfonamide-modified derivatives with the COX and cannabinoid (CB) receptors. Validation against benchmark data sets demonstrated the accuracy of these methods in predicting binding affinities while minimizing false positives and false negatives often associated with conventional docking tools. Our findings indicate that Δ9-THC and its carboxylic acid derivative exhibit strong binding affinities for COX-2 and CB2, suggesting their potential as anti-inflammatory agents, though their significant CB1 affinity suggests psychoactive risks. In contrast, carboxylic acid derivatives such as CBCA, CBNA, CBEA, CBTA, and CBLA demonstrated selective binding to COX-2 and CB2, with low CB1 affinity, supporting their potential as promising anti-inflammatory leads with reduced psychoactive side effects. Sulfonamide-modified analogs further enhanced COX-2 binding affinities and selectivity, displaying favorable drug-like properties, including compliance with Lipinski's rules, noninhibition of cytochromes P450, and oral bioavailability. These results highlight the utility of GFN2-xTB in identifying and optimizing cannabinoid-based therapeutic candidates for anti-inflammatory applications.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Brewer C.; Waddell D. Role of Prostaglandin F2 alpha in Skeletal Muscle Regeneration. J. Trainology 2012, 1, 45–52. 10.17338/trainology.1.2_45. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
