Xuanfei Baidu Decoction Alleviated Sepsis-Induced ALI by Modulating Gut Microbial Homeostasis and Promoting Inflammation Resolution: Bioinformatics and Experimental Study
- PMID: 40224467
- PMCID: PMC11983172
- DOI: 10.1021/acsomega.4c10575
Xuanfei Baidu Decoction Alleviated Sepsis-Induced ALI by Modulating Gut Microbial Homeostasis and Promoting Inflammation Resolution: Bioinformatics and Experimental Study
Abstract
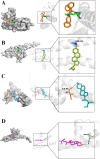
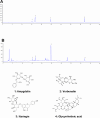
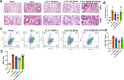
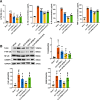
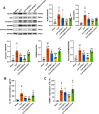
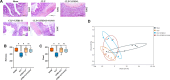
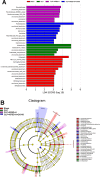
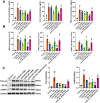
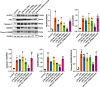
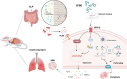
The Xuanfei Baidu Decoction (XFBD) has shown effective therapeutic potential for acute lung injury (ALI) induced by lipopolysaccharide and immunoglobin G immune complexes. Herein, the protective effects and mechanisms of XFBD were investigated in a sepsis-induced ALI mouse model along with its effects on gut microbiota. Notably, bioinformatics and molecular docking analyses revealed that XFBD components exhibited a strong binding affinity to G-protein-coupled receptor 18 (GPR18). In the murine ALI model-induced by cecal ligation and puncture (CLP)-XFBD markedly improved lung histopathology, reduced M1 macrophage polarization, and decreased pro-inflammatory cytokine levels in both lung tissues and MH-S macrophages. Furthermore, XFBD downregulated key inflammatory pathways, including nuclear factor (NF)-κB, phosphorylated-NF-κB, CCAAT/enhancer binding protein-δ, and the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3/Caspase-1/gasdermin D axis. Additionally, XFBD restored the CLP-induced disruption in gut microbiota balance, increasing the abundance of Prevotellaceae and Ruminococcaceae_UCG_014. Altogether, the findings of this study suggest that XFBD alleviates CLP-induced ALI by modulating gut microbial homeostasis and inhibiting associated inflammatory pathways, particularly via GPR18 activation, presenting the promising therapeutic potential of XFBD for treating sepsis-induced ALI.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Xuanfei Baidu Decoction suppresses complement overactivation and ameliorates IgG immune complex-induced acute lung injury by inhibiting JAK2/STAT3/SOCS3 and NF-κB signaling pathway.Phytomedicine. 2023 Jan;109:154551. doi: 10.1016/j.phymed.2022.154551. Epub 2022 Nov 21. Phytomedicine. 2023. PMID: 36610119 Free PMC article.
-
Xuanfei Baidu Formula attenuates LPS-induced acute lung injury by inhibiting the NF-κB signaling pathway.J Ethnopharmacol. 2023 Jan 30;301:115833. doi: 10.1016/j.jep.2022.115833. Epub 2022 Oct 14. J Ethnopharmacol. 2023. PMID: 36252879 Free PMC article.
-
Naringin dihydrochalcone alleviates sepsis-induced acute lung injury via improving gut microbial homeostasis and activating GPR18 receptor.Int Immunopharmacol. 2024 Aug 20;137:112418. doi: 10.1016/j.intimp.2024.112418. Epub 2024 Jun 18. Int Immunopharmacol. 2024. PMID: 38901244
-
Xuanfei Baidu Decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway.Pharmacol Res. 2022 Feb;176:106083. doi: 10.1016/j.phrs.2022.106083. Epub 2022 Jan 13. Pharmacol Res. 2022. PMID: 35033647 Free PMC article.
-
Xuanfei Baidu decoction in the treatment of coronavirus disease 2019 (COVID-19): Efficacy and potential mechanisms.Heliyon. 2023 Aug 19;9(9):e19163. doi: 10.1016/j.heliyon.2023.e19163. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809901 Free PMC article. Review.
References
-
- Butt Y.; Kurdowska A.; Allen T. C. Acute Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med. 2016, 140 (4), 345–350. 10.5858/arpa.2015-0519-RA. - DOI - PubMed
- Ragaller M.; Richter T. Acute lung injury and acute respiratory distress syndrome. J. Emerg Trauma Shock 2010, 3 (1), 43–51. 10.4103/0974-2700.58663. - DOI - PMC - PubMed
-
- Khilnani G. C.; Hadda V. Corticosteroids and ARDS: A review of treatment and prevention evidence. Lung India 2011, 28 (2), 114–119. 10.4103/0970-2113.80324. - DOI - PMC - PubMed
- Kuperminc E.; Heming N.; Carlos M.; Annane D. Corticosteroids in ARDS. J. Clin Med. 2023, 12, 3340.10.3390/jcm12093340. - DOI - PMC - PubMed
- Matuschak G. M.; Lechner A. J. Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. Mo Med. 2010, 107 (4), 252–258. - PMC - PubMed
-
- Cao L.; Wang Y.; Wang Y.; Lv F.; Liu L.; Li Z. Resolvin D2 suppresses NLRP3 inflammasome by promoting autophagy in macrophages. Exp Ther Med. 2021, 22 (5), 1222.10.3892/etm.2021.10656. - DOI - PMC - PubMed
- Sundarasivarao P. Y. K.; Walker J. M.; Rodriguez A.; Spur B. W.; Yin K. Resolvin D2 induces anti-microbial mechanisms in a model of infectious peritonitis and secondary lung infection. Front Immunol 2022, 13, 101194410.3389/fimmu.2022.1011944. - DOI - PMC - PubMed
- Chiang N.; de la Rosa X.; Libreros S.; Serhan C. N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol 2017, 198 (2), 842–851. 10.4049/jimmunol.1601650. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
