Integrating cuproptosis and immunosenescence: A novel therapeutic strategy in cancer treatment
- PMID: 40224540
- PMCID: PMC11986980
- DOI: 10.1016/j.bbrep.2025.101983
Integrating cuproptosis and immunosenescence: A novel therapeutic strategy in cancer treatment
Abstract
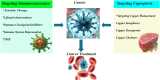
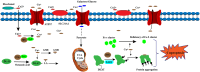
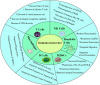
Recent advancements in our understanding of cell death mechanisms have progressed beyond traditional apoptosis to encompass various forms of regulated cell death, notably cuproptosis. This copper-dependent cell death occurs when copper interacts with lipoylated enzymes in the tricarboxylic acid cycle, leading to protein aggregation and subsequent cell death. Alongside this, immunosenescence the gradual decline in immune function due to aging has emerged as a significant factor in cancer progression and response to treatment. Innovative strategies that integrate cuproptosis and immunosenescence are showing considerable promise in cancer therapy. By leveraging the altered copper metabolism in cancer cells, cuproptosis can selectively induce cell death, effectively targeting and eliminating tumors. Simultaneously, addressing immunosenescence can rejuvenate the aging immune system, enhancing its capacity to identify and destroy cancer cells. This dual approach creates a synergistic effect, optimizing therapeutic efficacy by directly attacking tumor cells while revitalizing the immune response. Such integration bolsters the defense against cancer progression and recurrence and holds great potential for advancing cancer treatment modalities and improving patient outcomes. This paper delves into the interactions between cuproptosis and immunosenescence, emphasizing their implications for developing innovative cancer therapies.
Keywords: Cancer; Cuproptosis; Immunosenescence; Regulated cell death; Therapeutic resistance.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Copper metabolism and cuproptosis in human malignancies: Unraveling the complex interplay for therapeutic insights.Heliyon. 2024 Mar 7;10(5):e27496. doi: 10.1016/j.heliyon.2024.e27496. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38486750 Free PMC article. Review.
-
Copper homeostasis and cuproptosis in health and disease.Signal Transduct Target Ther. 2022 Nov 23;7(1):378. doi: 10.1038/s41392-022-01229-y. Signal Transduct Target Ther. 2022. PMID: 36414625 Free PMC article. Review.
-
Copper homeostasis and cuproptosis in cancer immunity and therapy.Immunol Rev. 2024 Jan;321(1):211-227. doi: 10.1111/imr.13276. Epub 2023 Sep 16. Immunol Rev. 2024. PMID: 37715546 Review.
-
Cuproptosis-related genes and agents: implications in tumor drug resistance and future perspectives.Front Pharmacol. 2025 May 8;16:1559236. doi: 10.3389/fphar.2025.1559236. eCollection 2025. Front Pharmacol. 2025. PMID: 40406488 Free PMC article. Review.
-
Cuproptosis in cancer: biological implications and therapeutic opportunities.Cell Mol Biol Lett. 2024 Jun 25;29(1):91. doi: 10.1186/s11658-024-00608-3. Cell Mol Biol Lett. 2024. PMID: 38918694 Free PMC article. Review.
Cited by
-
Cuproptosis: a novel therapeutic mechanism in lung cancer.Cancer Cell Int. 2025 Jun 24;25(1):231. doi: 10.1186/s12935-025-03864-1. Cancer Cell Int. 2025. PMID: 40555995 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

