Targeting PAR-2-driven inflammatory pathways in colorectal cancer: mechanistic insights from atorvastatin and rosuvastatin treatment in cell line models
- PMID: 40224964
- PMCID: PMC11985218
- DOI: 10.21037/tcr-24-1027
Targeting PAR-2-driven inflammatory pathways in colorectal cancer: mechanistic insights from atorvastatin and rosuvastatin treatment in cell line models
Abstract
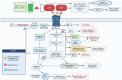
Background: Colorectal cancer (CRC) is a growing health concern globally and in regions such as the United Arab Emirates, where risk factors like obesity and hyperlipidaemia are prevalent. Chronic inflammation, driven by pathways involving protease-activated receptor 2 (PAR-2), plays a pivotal role in CRC progression, creating a tumour-promoting microenvironment. The overexpression of PAR-2 has been associated with increased tumour aggressiveness and drug resistance. While previous studies have focused on broad inflammatory modulation, this study explores the selective targeting of PAR-2 by atorvastatin (ATV) and rosuvastatin (RSV), highlighting their specificity by assessing minimal impact on PAR-1 expression, which serves as a control.
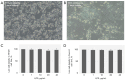
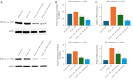
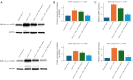
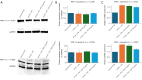
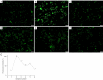
Methods: HT-29 and Caco-2 CRC cell lines were employed to investigate the anti-inflammatory effects of ATV and RSV. Inflammation was induced with lipopolysaccharide (LPS), followed by treatment with varying concentrations of ATV and RSV. Western blotting and real-time polymerase chain reaction for quantification (qPCR) were performed to quantify PAR-2 and TNF-α at both the protein and mRNA levels. Enzyme linked immunosorbent assay (ELISA) was used to measure the secretion of TNF-α. Calcium signalling, which plays a crucial role in inflammation, was analysed using Fluo-4 AM dye, with fluorescence imaging capturing the effects of statin treatment on intracellular calcium influx.
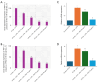
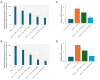
Results: LPS treatment significantly upregulated PAR-2 and TNF-α expression in both cell lines, validating the inflammatory model. Co-treatment with ATV or RSV reduced PAR-2 and TNF-α expression in a dose-dependent manner. The higher concentrations of ATV (50 µg/mL) and RSV (20 µg/mL) produced the most significant reduction in these inflammatory markers at both the protein and mRNA levels. Importantly, the treatment did not substantially alter PAR-1 expression, underlining the specificity of ATV and RSV in modulating PAR-2-mediated pathways. Additionally, statin treatment attenuated LPS-induced calcium influx, with fluorescence intensity decreasing markedly at higher concentrations of both statins.
Conclusions: This study provides novel insights into the selective targeting of PAR-2 by ATV and RSV, distinguishing their effects from PAR-1. The reduction in PAR-2 expression and TNF-α secretion, along with the suppression of calcium signalling, underscores the potential of these statins as targeted anti-inflammatory agents in CRC. The findings highlight the therapeutic value of ATV and RSV in modulating inflammation through PAR-2-specific pathways, which may contribute to reduced cancer progression. These results pave the way for further preclinical and clinical evaluations to explore statins as adjunctive therapies in the management of CRC.
Keywords: Protease-activated receptor 2 (PAR-2); calcium signalling; colorectal cancer (CRC); inflammation; statins.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1027/coif). The authors have no conflicts of interest to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
