Scoparone suppresses proliferation and cell cycle of hepatocellular carcinoma cells via inhibiting AKT/GSK-3β/cyclin D1 signaling pathway
- PMID: 40224969
- PMCID: PMC11985190
- DOI: 10.21037/tcr-24-1771
Scoparone suppresses proliferation and cell cycle of hepatocellular carcinoma cells via inhibiting AKT/GSK-3β/cyclin D1 signaling pathway
Abstract
Background: Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent cancer and the fourth leading cause of cancer-related mortality globally. Scoparone, a natural coumarin derivative primarily derived from Artemisia Capillaris Thunb, has demonstrated antitumor properties across various cancer types. However, its functions in HCC have not been clearly elucidated. This study aimed to investigate the antitumor effects of scoparone on the MHCC-97L and HCCC-9810 HCC cell lines.
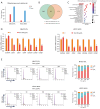
Methods: Cell proliferation was assessed through viability and colony formation assays. Migration and invasion capabilities of the cells were evaluated by wound healing assays and Transwell assays. Additionally, transcriptome sequencing and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were conducted to uncover pathways linked to gene enrichment in the artemisinin treatment group. Western blotting and flow cytometry were utilized to analyze the expression of mechanistic proteins associated with artemisinin treatment in HCC.
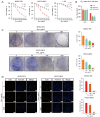
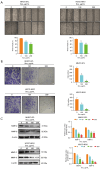
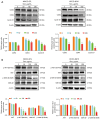
Results: Our findings revealed that scoparone effectively inhibited the proliferation, migration, and invasion of HCC cells. The genes affected by scoparone treatment were predominantly enriched in pathways related to the cell cycle. Specifically, scoparone reduced the expression of genes such as CDK2, CDK3, CDK4, CDC25A, CCND1, and CCNE1, while it increased the expression of CDKN1A (p21). Furthermore, scoparone suppressed the levels of cell cycle-related proteins CDK2, CDK4, and cyclin D1, along with the signaling pathways involving p-AKT and p-GSK-3β. Notably, the inhibitory effects of scoparone on HCC cell proliferation were partially reversed by the AKT activator, SC79.
Conclusions: Scoparone inhibited HCC cell viability by targeting the AKT/GSK-3β/cyclin D1 pathway.
Keywords: AKT/GSK-3β/cyclin D1 pathway; Hepatocellular carcinoma (HCC); cell cycle; scoparone.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1771/coif). The authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
