Obinutuzumab and Ofatumumab are More Effective Than Rituximab in the Treatment of Membranous Nephropathy Patients With Anti-Rituximab Antibodies
- PMID: 40225374
- PMCID: PMC11993203
- DOI: 10.1016/j.ekir.2024.12.012
Obinutuzumab and Ofatumumab are More Effective Than Rituximab in the Treatment of Membranous Nephropathy Patients With Anti-Rituximab Antibodies
Abstract
Introduction: Although rituximab has significantly improved outcomes for patients with membranous nephropathy, response to treatment is not universal and drug resistance can occur. One mechanism of resistance is the occurrence of antidrug antibodies. Obinutuzumab and ofatumumab are humanized and human monoclonal antibodies, respectively, that target B cells. These treatments have been shown to be effective in membranous nephropathy. However, obinutuzumab and ofatumumab have never been compared with rituximab in the treatment of patients with membranous nephropathy with anti-rituximab antibodies. We aimed to compare the efficacy and safety of obinutuzumab and ofatumumab with rituximab in patients with membranous nephropathy with anti-rituximab antibodies.
Methods: This international retrospective multicenter study enrolled 34 patients with membranous nephropathy from 5 nephrology departments in France, India, and Italy. All the patients had previously developed anti-rituximab antibodies. Nineteen patients received rituximab, 12 received obinutuzumab, and 3 received ofatumumab.
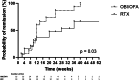
Results: Patients treated with obinutuzumab or ofatumumab were more likely to achieve clinical remission than those treated with rituximab at month 6 (87% vs. 37%, P = 0.005) and month 12 (87% vs. 42%, P = 0.01). Patients treated with obinutuzumab or ofatumumab were more likely to achieve immunological remission and B-cell depletion at month 6 than the patients treated with rituximab (92% vs. 56%, P = 0.04 and 93% vs. 35%, P = 0.002, respectively). No serious adverse events were reported in the obinutuzumab or ofatumumab group.
Conclusion: Obinutuzumab and ofatumumab are more effective than rituximab in treating patients with membranous nephropathy with anti-rituximab antibodies. Anti-rituximab antibodies should be systematically monitored, to determine appropriate treatment.
Keywords: antidrug antibodies; immunomonitoring; membranous nephropathy; obinutuzumab; ofatumumab; rituximab.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
LinkOut - more resources
Full Text Sources

